The Microbiological Burden of Short-Term Catheter Reuse in Individuals with Spinal Cord Injury: A Prospective Study
- PMID: 37509568
- PMCID: PMC10377649
- DOI: 10.3390/biomedicines11071929
The Microbiological Burden of Short-Term Catheter Reuse in Individuals with Spinal Cord Injury: A Prospective Study
Abstract
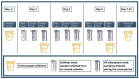
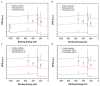
Despite the risk of developing catheter-associated urinary tract infections (CAUTI), catheter reuse is common among people with spinal cord injury (SCI). This study examined the microbiological burden and catheter surface changes associated with short-term reuse. Ten individuals with chronic SCI reused their catheters over 3 days. Urine and catheter swab cultures were collected daily for analysis. Scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS) analyses were used to assess catheter surface changes. Catheter swab cultures showed no growth after 48 h (47.8%), skin flora (28.9%), mixed flora (17.8%), or bacterial growth (5.5%). Asymptomatic bacteriuria was found for most participants at baseline (n = 9) and all at follow-up (n = 10). Urine samples contained Escherichia coli (58%), Klebsiella pneumoniae (30%), Enterococcus faecalis (26%), Acinetobacter calcoaceticus-baumannii (10%), Pseudomonas aeruginosa (6%) or Proteus vulgaris (2%). Most urine cultures showed resistance to one or more antibiotics (62%). SEM images demonstrated structural damage, biofilm and/or bacteria on all reused catheter surfaces. XPS analyses also confirmed the deposition of bacterial biofilm on reused catheters. Catheter surface changes and the presence of antibiotic-resistant bacteria were evident following short-term reuse, which may increase susceptibility to CAUTI in individuals with SCI despite asymptomatic bacteriuria.
Keywords: asymptomatic colonization; bacteria; electron microscopy; intermittent urethral catheterization; lower urinary tract symptoms; neurogenic urinary bladder disorder; photoelectron spectroscopy; spinal cord injury; urinary tract infections.
Conflict of interest statement
M.W. receives or has received research support from the Rick Hansen Institute and Foundation, the Michael Smith Foundation for Health Research, Pfizer Canada, Coloplast, and Wellspect. M.W. serves on the advisory board for Coloplast. A.V.K. receives or has received research support from the Craig Neilsen Foundation, the Rick Hansen Institute and Foundation, the Canadian Institutes of Health Research, the Canadian Foundation for Innovation, the Heart and Stroke Foundation of Canada, the Michael Smith Foundation for Health Research, the Minnesota Spinal Cord Injury and Traumatic Brain Injury Research Grant Program, Wings For Life Spinal Cord Research Foundation, Pfizer, Allergan, Coloplast, and Purdue; serves on advisory boards for Coloplast, Wellspect, and the Craig H. Neilsen Foundation; and is Past-President of the American Spinal Injury Association (ASIA). All other authors have no conflict of interest to declare.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

