The Potential Influence of Uremic Toxins on the Homeostasis of Bones and Muscles in Chronic Kidney Disease
- PMID: 37509715
- PMCID: PMC10377042
- DOI: 10.3390/biomedicines11072076
The Potential Influence of Uremic Toxins on the Homeostasis of Bones and Muscles in Chronic Kidney Disease
Abstract
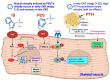
Patients with chronic kidney disease (CKD) often experience a high accumulation of protein-bound uremic toxins (PBUTs), specifically indoxyl sulfate (IS) and p-cresyl sulfate (pCS). In the early stages of CKD, the buildup of PBUTs inhibits bone and muscle function. As CKD progresses, elevated PBUT levels further hinder bone turnover and exacerbate muscle wasting. In the late stage of CKD, hyperparathyroidism worsens PBUT-induced muscle damage but can improve low bone turnover. PBUTs play a significant role in reducing both the quantity and quality of bone by affecting osteoblast and osteoclast lineage. IS, in particular, interferes with osteoblastogenesis by activating aryl hydrocarbon receptor (AhR) signaling, which reduces the expression of Runx2 and impedes osteoblast differentiation. High PBUT levels can also reduce calcitriol production, increase the expression of Wnt antagonists (SOST, DKK1), and decrease klotho expression, all of which contribute to low bone turnover disorders. Furthermore, PBUT accumulation leads to continuous muscle protein breakdown through the excessive production of reactive oxygen species (ROS) and inflammatory cytokines. Interactions between muscles and bones, mediated by various factors released from individual tissues, play a crucial role in the mutual modulation of bone and muscle in CKD. Exercise and nutritional therapy have the potential to yield favorable outcomes. Understanding the underlying mechanisms of bone and muscle loss in CKD can aid in developing new therapies for musculoskeletal diseases, particularly those related to bone loss and muscle wasting.
Keywords: bone loss; chronic kidney disease; indoxyl sulfate; sarcopenia; uremic toxins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The influence of uremic toxins on low bone turnover disease in chronic kidney disease.Tzu Chi Med J. 2023 Dec 13;36(1):38-45. doi: 10.4103/tcmj.tcmj_212_23. eCollection 2024 Jan-Mar. Tzu Chi Med J. 2023. PMID: 38406573 Free PMC article. Review.
-
Protein-bound uremic toxins (PBUTs) in chronic kidney disease (CKD) patients: Production pathway, challenges and recent advances in renal PBUTs clearance.NanoImpact. 2021 Jan;21:100299. doi: 10.1016/j.impact.2021.100299. Epub 2021 Jan 28. NanoImpact. 2021. PMID: 35559786 Review.
-
Uremic Toxins and Frailty in Patients with Chronic Kidney Disease: A Molecular Insight.Int J Mol Sci. 2021 Jun 10;22(12):6270. doi: 10.3390/ijms22126270. Int J Mol Sci. 2021. PMID: 34200937 Free PMC article. Review.
-
Protein-Bound Uremic Toxins in Hemodialysis Patients Relate to Residual Kidney Function, Are Not Influenced by Convective Transport, and Do Not Relate to Outcome.Toxins (Basel). 2020 Apr 7;12(4):234. doi: 10.3390/toxins12040234. Toxins (Basel). 2020. PMID: 32272776 Free PMC article. Clinical Trial.
-
Effect of uremic toxin-indoxyl sulfate on the skeletal system.Clin Chim Acta. 2018 Sep;484:197-206. doi: 10.1016/j.cca.2018.05.057. Epub 2018 Jun 1. Clin Chim Acta. 2018. PMID: 29864403 Review.
Cited by
-
Co-Housing and Fecal Microbiota Transplantation: Technical Support for TCM Herbal Treatment of Extra-Intestinal Diseases Based on Gut Microbial Ecosystem Remodeling.Drug Des Devel Ther. 2023 Dec 24;17:3803-3831. doi: 10.2147/DDDT.S443462. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 38155743 Free PMC article. Review.
-
The influence of uremic toxins on low bone turnover disease in chronic kidney disease.Tzu Chi Med J. 2023 Dec 13;36(1):38-45. doi: 10.4103/tcmj.tcmj_212_23. eCollection 2024 Jan-Mar. Tzu Chi Med J. 2023. PMID: 38406573 Free PMC article. Review.
-
Skeletal Muscle Injury in Chronic Kidney Disease-From Histologic Changes to Molecular Mechanisms and to Novel Therapies.Int J Mol Sci. 2024 May 8;25(10):5117. doi: 10.3390/ijms25105117. Int J Mol Sci. 2024. PMID: 38791164 Free PMC article. Review.
-
Hyperphosphatemia Contributes to Skeletal Muscle Atrophy in Mice.Int J Mol Sci. 2024 Aug 28;25(17):9308. doi: 10.3390/ijms25179308. Int J Mol Sci. 2024. PMID: 39273260 Free PMC article.
-
Molecular and Cellular Mechanisms Linking Chronic Kidney Disease and Sarcopenia in Aging: An Integrated Perspective.Clin Interv Aging. 2025 Apr 8;20:449-458. doi: 10.2147/CIA.S516704. eCollection 2025. Clin Interv Aging. 2025. PMID: 40226833 Free PMC article. Review.
References
-
- Holle J., Querfeld U., Kirchner M., Anninos A., Okun J., Thurn-Valsassina D., Bayazit A., Niemirska A., Canpolat N., Bulut I.K., et al. Indoxyl sulfate associates with cardiovascular phenotype in children with chronic kidney disease. Pediatr. Nephrol. 2019;34:2571–2582. doi: 10.1007/s00467-019-04331-6. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

