Bipolar Androgen Therapy: When Excess Fuel Extinguishes the Fire
- PMID: 37509723
- PMCID: PMC10377678
- DOI: 10.3390/biomedicines11072084
Bipolar Androgen Therapy: When Excess Fuel Extinguishes the Fire
Abstract
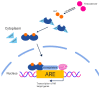
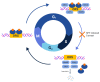
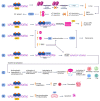
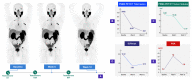
Androgen deprivation therapy (ADT) remains the cornerstone of advanced prostate cancer treatment. However, the progression towards castration-resistant prostate cancer is inevitable, as the cancer cells reactivate androgen receptor signaling and adapt to the castrate state through autoregulation of the androgen receptor. Additionally, the upfront use of novel hormonal agents such as enzalutamide and abiraterone acetate may result in long-term toxicities and may trigger the selection of AR-independent cells through "Darwinian" treatment-induced pressure. Therefore, it is crucial to develop new strategies to overcome these challenges. Bipolar androgen therapy (BAT) is one such approach that has been devised based on studies demonstrating the paradoxical inhibitory effects of supraphysiologic testosterone on prostate cancer growth, achieved through a variety of mechanisms acting in concert. BAT involves rapidly alternating testosterone levels between supraphysiological and near-castrate levels over a period of a month, achieved through monthly intramuscular injections of testosterone plus concurrent ADT. BAT is effective and well-tolerated, improving quality of life and potentially re-sensitizing patients to previous hormonal therapies after progression. By exploring the mechanisms and clinical evidence for BAT, this review seeks to shed light on its potential as a promising new approach to prostate cancer treatment.
Keywords: bipolar androgen therapy (BAT); metastatic castration-resistant prostate cancer; novel hormonal agents (NHAs); positron emission tomography (PET); prostate cancer; prostate-specific membrane antigen; supraphysiologic testosterone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Mohler J.L., Antonarakis E.S., Armstrong A.J., D’Amico A.V., Davis B.J., Dorff T., Eastham J.A., Enke C.A., Farrington T.A., Higano C.S., et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019;17:479–505. doi: 10.6004/jnccn.2019.0023. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

