Breaking through Multiple Myeloma: A Paradigm for a Comprehensive Tumor Ecosystem Targeting
- PMID: 37509726
- PMCID: PMC10377041
- DOI: 10.3390/biomedicines11072087
Breaking through Multiple Myeloma: A Paradigm for a Comprehensive Tumor Ecosystem Targeting
Abstract
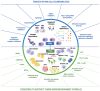
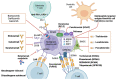
Multiple myeloma (MM) is a cancerous condition characterized by the proliferation of plasma cells within the hematopoietic marrow, resulting in multiple osteolytic lesions. MM patients typically experience bone pain, kidney damage, fatigue due to anemia, and infections. Historically, MM was an incurable disease with a life expectancy of around three years after diagnosis. However, over the past two decades, the development of novel therapeutics has significantly improved patient outcomes, including response to treatment, remission duration, quality of life, and overall survival. These advancements include thalidomide and its derivatives, lenalidomide and pomalidomide, which exhibit diverse mechanisms of action against the plasma cell clone. Additionally, proteasome inhibitors such as bortezomib, ixazomib, and carfilzomib disrupt protein degradation, proving specifically toxic to cancerous plasma cells. Recent advancements also involve monoclonal antibodies targeting surface antigens, such as elotuzumab (anti-CS1) and daratumumab (anti-CD38), bispecific t-cell engagers such as teclistamab (anti-BCMA/CD3) and Chimeric antigen receptor T (CAR-T)-based strategies, with a growing focus on drugs that exhibit increasingly targeted action against neoplastic plasma cells and relevant effects on the tumor microenvironment.
Keywords: immunotherapy; microenvironment; monoclonal antibody; multiple myeloma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Targeting B Cell Maturation Antigen (BCMA) in Multiple Myeloma: Potential Uses of BCMA-Based Immunotherapy.Front Immunol. 2018 Aug 10;9:1821. doi: 10.3389/fimmu.2018.01821. eCollection 2018. Front Immunol. 2018. PMID: 30147690 Free PMC article. Review.
-
Role of Immunotherapy in Targeting the Bone Marrow Microenvironment in Multiple Myeloma: An Evolving Therapeutic Strategy.Pharmacotherapy. 2017 Jan;37(1):129-143. doi: 10.1002/phar.1871. Epub 2017 Jan 6. Pharmacotherapy. 2017. PMID: 27870103 Review.
-
Novel CS1 CAR-T Cells and Bispecific CS1-BCMA CAR-T Cells Effectively Target Multiple Myeloma.Biomedicines. 2021 Oct 9;9(10):1422. doi: 10.3390/biomedicines9101422. Biomedicines. 2021. PMID: 34680541 Free PMC article.
-
Contemporary drug therapies for multiple myeloma.Drugs Today (Barc). 2013 Sep;49(9):563-73. doi: 10.1358/dot.2013.49.9.2020941. Drugs Today (Barc). 2013. PMID: 24086952 Review.
-
How First-Line Therapy is Changing in Transplant-Eligible Multiple Myeloma Patients.Mediterr J Hematol Infect Dis. 2025 Mar 1;17(1):e2025026. doi: 10.4084/MJHID.2025.026. eCollection 2025. Mediterr J Hematol Infect Dis. 2025. PMID: 40084095 Free PMC article. Review.
Cited by
-
Advancements in the Treatment of Multiple Myeloma.Cureus. 2024 Dec 2;16(12):e74970. doi: 10.7759/cureus.74970. eCollection 2024 Dec. Cureus. 2024. PMID: 39744254 Free PMC article. Review.
-
Targeting mTOR signaling pathways in multiple myeloma: biology and implication for therapy.Cell Commun Signal. 2024 Jun 11;22(1):320. doi: 10.1186/s12964-024-01699-3. Cell Commun Signal. 2024. PMID: 38862983 Free PMC article. Review.
-
Equecabtagene Autoleucel: First Approval.Mol Diagn Ther. 2023 Nov;27(6):781-787. doi: 10.1007/s40291-023-00673-y. Epub 2023 Sep 2. Mol Diagn Ther. 2023. PMID: 37658205 Review.
References
-
- Leuraud K., Richardson D.B., Cardis E., Daniels R.D., Gillies M., O’Hagan J.A., Hamra G.B., Haylock R., Laurier D., Moissonnier M., et al. Ionising Radiation and Risk of Death from Leukaemia and Lymphoma in Radiation-Monitored Workers (INWORKS): An International Cohort Study. Lancet Haematol. 2015;2:e276–e281. doi: 10.1016/S2352-3026(15)00094-0. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

