Mitochondrial Dysfunction in Peripheral Blood Mononuclear Cells as Novel Diagnostic Tools for Non-Alcoholic Fatty Liver Disease: Visualizing Relationships with Known and Potential Disease Biomarkers
- PMID: 37510108
- PMCID: PMC10378438
- DOI: 10.3390/diagnostics13142363
Mitochondrial Dysfunction in Peripheral Blood Mononuclear Cells as Novel Diagnostic Tools for Non-Alcoholic Fatty Liver Disease: Visualizing Relationships with Known and Potential Disease Biomarkers
Abstract
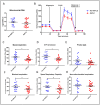
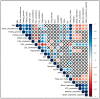
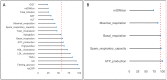
Non-alcoholic fatty liver disease (NAFLD) is a health emergency worldwide due to its high prevalence and the lack of specific therapies. Noninvasive biomarkers supporting NAFLD diagnosis are urgently needed. Liver mitochondrial dysfunction is a central NAFLD pathomechanism that changes throughout disease progression. Blood-cell bioenergetics reflecting mitochondrial organ dysfunction is emerging for its potential applications in diagnostics. We measured real-time mitochondrial respirometry in peripheral blood mononuclear cells (PBMCs), anthropometric parameters, routine blood analytes, and circulating cytokines from a cohort of NAFLD patients (N = 19) and non-NAFLD control subjects (N = 18). PBMC basal respiration, ATP-linked respiration, maximal respiration, and spare respiratory capacity were significantly reduced in NAFLD compared to non-NAFLD cases. Correlation plots were applied to visualize relationships between known or potential NAFLD-related biomarkers, while non-parametric methods were applied to identify which biomarkers are NAFLD predictors. Basal and ATP-linked mitochondrial respiration were negatively correlated with triglycerides and fasting insulin levels and HOMA index. Maximal and spare respiratory capacity were negatively correlated with IL-6 levels. All the mitochondrial respiratory parameters were positively correlated with HDL-cholesterol level and negatively correlated with fatty liver index. We propose including blood cell respirometry in panels of NAFLD diagnostic biomarkers to monitor disease progression and the response to current and novel therapies, including mitochondrial-targeted ones.
Keywords: correlation plot; mitochondrial bioenergetics; non-alcoholic fatty liver disease; peripheral blood mononuclear cells; random forest; relative variable importance.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL–EASD–EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

