A Real-World Multicenter Retrospective Observational Study on Polish Experience with Nintedanib Therapy in Patients with Idiopathic Pulmonary Fibrosis: The PolExNIB Study
- PMID: 37510750
- PMCID: PMC10381008
- DOI: 10.3390/jcm12144635
A Real-World Multicenter Retrospective Observational Study on Polish Experience with Nintedanib Therapy in Patients with Idiopathic Pulmonary Fibrosis: The PolExNIB Study
Abstract
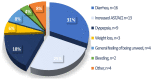
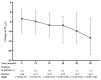
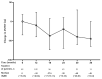
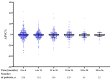
Nintedanib is a disease-modifying agent licensed for the treatment of IPF. Data on Polish experience with nintedanib in IPF are lacking. The present study aimed to describe the safety and efficacy profiles of nintedanib in a large real-world cohort of Polish patients with IPF. This was a multicenter, retrospective, observational study of IPF patients treated with nintedanib between March 2018 and October 2021. Data collection included baseline clinical characteristics, results of pulmonary function tests (PFTs), and a six-minute walk test (6MWT). Longitudinal data on PFTs, 6MWT, adverse drug reactions (ADRs), and treatment persistence were also retrieved. A total of 501 patients (70% male) with a median age of 70.9 years (IQR 65-75.7) were included in this study. Patients were followed on treatment for a median of 15 months (7-25.5). The majority of patients (66.7%) were treated with the full recommended dose of nintedanib and 33.3% of patients were treated with a reduced dose of a drug. Intermittent dose reductions or drug interruptions were needed in 20% of patients. Over up to 3 years of follow-up, pulmonary function remained largely stable with the minority experiencing disease progression. The most frequent ADRs included diarrhea (45.3%), decreased appetite (29.9%), abdominal discomfort (29.5%), weight loss (32.1%), nausea (20.8%), fatigue (19.2%), increased liver aminotransferases (15.4%), and vomiting (8.2%). A total of 203 patients (40.5%) discontinued nintedanib treatment due to diverse reasons including ADRs (10.2%), death (11.6%), disease progression (4.6%), patient's request (6.6%), and neoplastic disease (2.2%). This real-world study of a large cohort of Polish patients with IPF demonstrates that nintedanib therapy is safe, and is associated with acceptable tolerance and disease stabilization. These data support the findings of previously conducted clinical trials and observational studies on the safety and efficacy profiles of nintedanib in IPF.
Keywords: Poland; efficacy; idiopathic pulmonary fibrosis (IPF); nintedanib; real-world data; safety.
Conflict of interest statement
S.M. declares receiving grants and personal fees from Boehringer Ingelheim and Roche outside of submitted work. A.J.B. declares receiving grants from Boehringer Ingelheim and Roche outside of submitted work. A.B. declares receiving grants and personal fees from Boehringer Ingelheim outside of submitted work. H.B-G. declares no conflict of interest related to this work. M.B. declares receiving personal fees from Boehringer Ingelheim and Roche outside of submitted work. A.D. declares receiving personal fees from Boehringer Ingelheim outside of submitted work. K.G. declares receiving grants and personal fees from Boehringer Ingelheim and Roche outside of submitted work. L.G.-S. declares receiving grants and personal fees from Boehringer Ingelheim outside of submitted work. H.J.-L. declares receiving grants and personal fees from Boehringer Ingelheim outside of submitted work. A.J. declares receiving grants and personal fees from Boehringer Ingelheim and Roche outside of submitted work. E.J. declares no conflict of interest related to this work. D.J. declares receiving grants and personal fees from Boehringer Ingelheim and Roche outside of submitted work. A.K. declares receiving grants and personal fees from Boehringer Ingelheim and Roche outside of submitted work. M.K. declares receiving grants and personal fees from Roche outside of submitted work. M.K. declares receiving grants and personal fees from Boehringer Ingelheim and Roche outside of submitted work. R.K. declares receiving personal fees from Boehringer Ingelheim outside of submitted work. K.L. declares receiving grants and personal fees from Boehringer Ingelheim and Roche outside of submitted work. B.M. declares receiving grants and personal fees from Boehringer Ingelheim outside of submitted work. M.M.M.-B. declares receiving grants and personal fees from Boehringer Ingelheim outside of submitted work. J.M. declares no conflict of interest related to this work. M.N.-P. declares receiving grants and personal fees from Boehringer Ingelheim outside of submitted work. A.N. declares receiving grants and personal fees from Boehringer Ingelheim and Roche outside of submitted work. K.R.-Ś. declares no conflict of interest related to this work. A.S. declares receiving personal fees from Boehringer Ingelheim outside of submitted work. KS declares no conflict of interest related to this work. M.S. declares receiving grants and personal fees from Boehringer Ingelheim and Roche outside of submitted work. T.S. declares receiving grants and personal fees from Boehringer Ingelheim outside of submitted work. M.T. declares no conflict of interest related to this work. W.T. declares receiving grants and personal fees from Boehringer Ingelheim and Roche outside of submitted work. M.T.-S. declares no conflict of interest related to this work. D.Z. declares receiving grants and personal fees from Boehringer Ingelheim and Roche outside of submitted work. B.Ż. declares no conflict of interest related to this work. W.J.P. declares receiving grants and personal fees from Boehringer Ingelheim and Roche outside of submitted work.
Figures



References
-
- Raghu G., Remy-Jardin M., Myers J.L., Richeldi L., Ryerson C.J., Lederer D.J., Behr J., Cottin V., Danoff S.K., Morell F., et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. - DOI - PubMed
-
- Noble P.W., Albera C., Bradford W.Z., Costabel U., Glassberg M.K., Kardatzke D., King T.E., Lancaster L., Sahn S.A., Szwarcberg J., et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet Lond. Engl. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. - DOI - PubMed
-
- King T.E., Bradford W.Z., Castro-Bernardini S., Fagan E.A., Glaspole I., Glassberg M.K., Gorina E., Hopkins P.M., Kardatzke D., Lancaster L., et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. - DOI - PubMed
LinkOut - more resources
Full Text Sources

