Benefits of Paediatric to Adult Transition Programme in Inflammatory Bowel Disease: The BUTTERFLY Study of GETECCU and SEGHNP
- PMID: 37510928
- PMCID: PMC10381381
- DOI: 10.3390/jcm12144813
Benefits of Paediatric to Adult Transition Programme in Inflammatory Bowel Disease: The BUTTERFLY Study of GETECCU and SEGHNP
Abstract
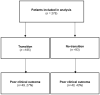
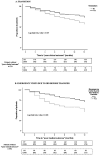
(1) Background: Transition is a planned movement of paediatric patients to adult healthcare systems, and its implementation is not yet established in all inflammatory bowel disease (IBD) units. The aim of the study was to evaluate the impact of transition on IBD outcomes. (2) Methods: Multicentre, retrospective and observational study of IBD paediatric patients transferred to an adult IBD unit between 2017-2020. Two groups were compared: transition (≥1 joint visit involving the gastroenterologist, the paediatrician, a programme coordinator, the parents and the patient) and no-transition. Outcomes within one year after transfer were analysed. The main variable was poor clinical outcome (IBD flare, hospitalisation, surgery or any change in the treatment because of a flare). Predictive factors of poor clinical outcome were identified with multivariable analysis. (3) Results: A total of 278 patients from 34 Spanish hospitals were included. One hundred eighty-five patients (67%) from twenty-two hospitals (65%) performed a structured transition. Eighty-nine patients had poor clinical outcome at one year after transfer: 27% in the transition and 43% in the no-transition group (p = 0.005). One year after transfer, no-transition patients were more likely to have a flare (36% vs. 22%; p = 0.018) and reported more hospitalisations (10% vs. 3%; p = 0.025). The lack of transition, as well as parameters at transfer, including IBD activity, body mass index < 18.5 and corticosteroid treatment, were associated with poor clinical outcome. One patient in the transition group (0.4%) was lost to follow-up. (4) Conclusion: Transition care programmes improve patients' outcomes after the transfer from paediatric to adult IBD units. Active IBD at transfer impairs outcomes.
Keywords: inflammatory bowel disease; transition; transitional care.
Conflict of interest statement
Cristina Rubín de Célix has received education funding from Ferring, Tillotts Pharma, AbbVie, Norgine, MSD, Pfizer, Takeda and Janssen. Javier Martín-de-Carpi has served as a speaker, consultant and advisory member for, or has received research funding from, AbbVie Adacyte, Abbott, Casen Recordati, Cellgene, Janssen, Mead Johnson, MSD, Norgine, Nutriben, Pfizer, Faes Farma, Ferring, Kern Pharma, Nestle, Lilly, Roche, Takeda, Tillotts and Dr. Falk Pharma. Gemma Pujol-Muncunill has served as a speaker or received education founding from AbbVie, Nestlé Health Science, Janssen, Nutricia and Pfizer. Laura María Palomino has received financial support for traveling and educational activities from AbbVie, Casen Recordati, Norgine, Nestlé, Mead Johnson, Ordesa, Nutricia and Abbott. Marta Velasco Rodríguez-Belvís has received financial support for traveling and educational activities, or served as speaker or consultant for AbbVie, Casen Recordati, Norgine, Nestlé, Mead Johnson, Ordesa, Nutricia and Abbott. María José Casanova has received research or education funding from Pfizer, Takeda, Janssen, MSD, Ferring, AbbVie, Biogen, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi and Norgine. Miriam Mañosa has served as a speaker, consultant and advisory member for, or has received research funding from, AbbVie, Galapagos, Janssen, MSD, Pfizer, Faes Farma, Takeda, Tillotts and Dr. Falk Pharma. Vicent Hernandez has served as consultant, has served as speaker, has received travel support or research funding from MSD, AbbVie, Ferring, Dr. Falk Pharma, Tillotts Pharma, Pfizer, Takeda, Janssen, Kern Pharma Biologics, Adacyte, Sandoz, Biogen and Fresenius-Kabi. Luis Menchén has served as a speaker, consultant and advisory member for, or has received research funding from, AbbVie, Galapagos, Janssen, MSD, Pfizer, Kern Pharma, Takeda, Tillotts, Ferring, Dr. Falk Pharma and General Electric. Manuel Barreiro-De Acosta has received financial support for traveling and educational activities from or has served as an advisory board member for Pfizer, MSD, Takeda, AbbVie, Kern Pharma, Janssen, Fresenius Kabi, Biogen, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Gebro Pharma, Otsuka Pharmaceuticals and Tillotts Pharma. Pilar Corsino has served as a speaker for, or has received research funding from, AbbVie, Janssen and Pfizer. Raquel Vicente has served as speaker, consultant and/or advisory member for, or has received research funding from, MSD, AbbVie, Pfizer, Takeda, Janssen, Ferring, Chiesi and Faes Farma. Estela Fernández-Salgado has served as speaker, consultant from AbbVie, Takeda, Janssen, Ferring and has received education funding from AbbVie, Pfizer, Takeda, Janssen, Tillotts Pharma, Dr. Falk Pharma, MSD, Faes Farma, Ferring. Sabino Riestra has served as speaker, consultant and advisory member for, or has received research funding from, MSD, AbbVie, Takeda, Janssen, Pfizer, Mylan, Biogen, Kern Pharma, Ferring, Faes Farma and Tillotts Pharma. Marta Calvo Moya has served as a speaker and consultant for, or has received research funding from, MSD, AbbVie, Takeda, Janssen, Ferring, Shire Pharmaceuticals, Tillotts Pharma, Dr. Falk Pharma and Gebro Pharma. Jose M. Huguet has served as a speaker and consultant for, or has received research funding from, MSD, AbbVie, Takeda, Janssen, Pfizer, Ferring, Sandoz, Tillotts Pharma, Dr. Falk Pharma and Faes Farma. Rocío Plaza has received education funding from AbbVie, Takeda, Janssen, Ferring, Pfizer, Tillotts Pharma and has served as a speaker for AbbVie, Takeda and Janssen. Iván Guerra has served as a speaker for Takeda and Sandoz. Teresa de Jesús Martínez-Pérez reports personal fees or nonfinancial support from Abbie, Amgen, MSD, Janssen, Takeda, Pfizer, Chiesi, Tillotts Pharma, Kern Pharma, outside the submitted work. María Calvo Iñiguez has received education funding from AbbVie, Takeda, Pfizer, Adacyte and Janssen. María Chaparro has served as a speaker, consultant and advisory member for, or received research funding from, AbbVie, Celltrion, Chiesi, Faes Farma, Dr. Falk Pharma, Ferring, Gebro Pharma, Janssen, MSD, Otsuka Pharmaceuticals, Pfizer Inc, Roche, Shire Pharmaceuticals, Takeda and Tillotts Pharma. Javier P. Gisbert has served as speaker, consultant and advisory member for, or has received research funding from, MSD, AbbVie, Pfizer, Kern Pharma, Biogen, Mylan, Takeda, Janssen, Roche, Sandoz, Celgene/Bristol Myers, Gilead/Galapagos, Lilly, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Norgine and Vifor Pharma. All other authors have nothing to declare.
Figures



References
-
- Blum R.W., Garell D., Hodgman C.H., Jorissen T.W., Okinow N.A., Orr D.P., Slap G.B. Transition from child-centered to adult health-care systems for adolescents with chronic conditions. A position paper of the Society for Adolescent Medicine. J. Adolesc. Health Off. Publ. Soc. Adolesc. Med. 1993;14:570–576. doi: 10.1016/1054-139X(93)90143-D. - DOI - PubMed
-
- Martin-de-Carpi J., Rodriguez A., Ramos E., Jimenez S., Martinez-Gomez M.J., Medina E., Navas-Lopez V.M., on behalf of the SPIRIT-IBD Working Group of SEGHNP The complete picture of changing pediatric inflammatory bowel disease incidence in Spain in 25 years (1985–2009): The EXPERIENCE registry. J. Crohn’s Colitis. 2014;8:763–769. doi: 10.1016/j.crohns.2014.01.005. - DOI - PubMed
-
- Kuenzig M.E., Fung S.G., Marderfeld L., Mak J.W.Y., Kaplan G.G., Ng S.C., Wilson D.C., Cameron F., Henderson P., Kotze P.G., et al. Twenty-first Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology. 2022;162:1147–1159.e4. doi: 10.1053/j.gastro.2021.12.282. - DOI - PubMed
-
- Brooks A.J., Smith P.J., Cohen R., Collins P., Douds A., Forbes V., Gaya D.R., Johnston B.T., McKiernan P.J., Murray C.D., et al. UK guideline on transition of adolescent and young persons with chronic digestive diseases from paediatric to adult care. Gut. 2017;66:988–1000. doi: 10.1136/gutjnl-2016-313000. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

