Factors Associated with Response to Systemic Corticosteroids in Active Ulcerative Colitis: Results from a Prospective, Multicenter Trial
- PMID: 37510968
- PMCID: PMC10382050
- DOI: 10.3390/jcm12144853
Factors Associated with Response to Systemic Corticosteroids in Active Ulcerative Colitis: Results from a Prospective, Multicenter Trial
Abstract
Background: Among patients with ulcerative colitis, 30-50% receive corticosteroids within the first five years after diagnosis. We aimed to reconsider their effectiveness in the context of the biologic era.
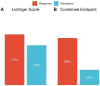
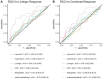
Methods: In this prospective, multicenter study, patients with active ulcerative colitis (Lichtiger score ≥ 4) were eligible if initiating systemic corticosteroids. The primary endpoint was clinical response (decrease in the Lichtiger score of ≥50%) at week 4. Secondary endpoints included combined response defined as clinical response and any reduction in elevated biomarkers (CRP and/or calprotectin). Steroid dependence was assessed after three months.
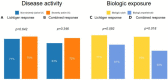
Results: A total of 103 patients were included. Clinical response was achieved by 73% of patients, and combined response by 68%. A total of 15% of patients were steroid-dependent. Activity of colitis did not influence short-term response to treatment but increased the risk for steroid dependence. Biologic-naïve patients responded better than biologic-experienced patients. Past smoking history (OR 5.38 [1.71, 20.1], p = 0.003), hemoglobin levels (OR 0.76 [0.57, 0.99] for higher levels, p = 0.045), and biologic experience (OR 3.30 [1.08, 10.6], p = 0.036) were independently associated with nonresponse.
Conclusion: Disease activity was not associated with short-term response to systemic corticosteroids but was associated with steroid dependence in patients with active ulcerative colitis. Exposure to biologics negatively affects response rates.
Keywords: inflammatory bowel disease; systemic corticosteroids; ulcerative colitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Jeuring S.F.G., Biemans V.B.C., van den Heuvel T.R.A., Zeegers M.P., Hameeteman W.H., Romberg-Camps M.J.L., Oostenbrug L.E., Masclee A.A.M., Jonkers D., Pierik M.J. Corticosteroid Sparing in Inflammatory Bowel Disease is More Often Achieved in the Immunomodulator and Biological Era-Results from the Dutch Population-Based IBDSL Cohort. Am. J. Gastroenterol. 2018;113:384–395. doi: 10.1038/ajg.2017.482. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

