A Novel Rat Model of ADHD-like Hyperactivity/Impulsivity after Delayed Reward Has Selective Loss of Dopaminergic Neurons in the Right Ventral Tegmental Area
- PMID: 37511013
- PMCID: PMC10379272
- DOI: 10.3390/ijms241411252
A Novel Rat Model of ADHD-like Hyperactivity/Impulsivity after Delayed Reward Has Selective Loss of Dopaminergic Neurons in the Right Ventral Tegmental Area
Abstract
In attention deficit hyperactivity disorder (ADHD), hyperactivity and impulsivity occur in response to delayed reward. Herein we report a novel animal model in which male Sprague-Dawley rats exposed to repeated hypoxic brain injury during the equivalent of extreme prematurity were ADHD-like hyperactive/impulsive in response to delayed reward and attentive at 3 months of age. Thus, a unique animal model of one of the presentations/subtypes of ADHD was discovered. An additional finding is that the repeated hypoxia rats were not hyperactive in the widely used open field test, which is not ADHD specific. Hence, it is recommended that ADHD-like hyperactivity and ADHD-like impulsivity, specifically in response to delayed reward, be a primary component in the design of future experiments that characterize potential animal models of ADHD, replacing open field testing of hyperactivity. Unknown is whether death and/or activity of midbrain dopaminergic neurons contributed to the ADHD-like hyperactivity/impulsivity detected after delayed reward. Hence, we stereologically measured the absolute number of dopaminergic neurons in four midbrain subregions and the average somal/nuclear volume of those neurons. Repeated hypoxia rats had a significant specific loss of dopaminergic neurons in the right ventral tegmental area (VTA) at 2 weeks of age and 18 months of age, providing new evidence of a site of pathology. No dopaminergic neuronal loss occurred in three other midbrain regions. Fewer VTA dopaminergic neurons correlated with increased ADHD-like hyperactivity and impulsivity. Novel early intervention therapies to rescue VTA dopaminergic neurons and potentially prevent ADHD-like hyperactivity/impulsivity can now be investigated.
Keywords: ADHD hyperactive/impulsive presentation; fixed-interval extinction test; midbrain dopaminergic neurons; open field test; stereology; ventral tegmental area.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
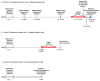



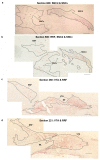


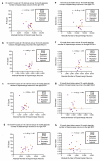

References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-V. 5th ed. American Psychiatric Association; Arlington, VA, USA: 2013.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

