Melanin's Journey from Melanocytes to Keratinocytes: Uncovering the Molecular Mechanisms of Melanin Transfer and Processing
- PMID: 37511054
- PMCID: PMC10379423
- DOI: 10.3390/ijms241411289
Melanin's Journey from Melanocytes to Keratinocytes: Uncovering the Molecular Mechanisms of Melanin Transfer and Processing
Abstract
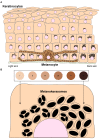
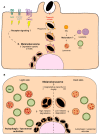
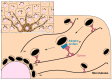
Skin pigmentation ensures efficient photoprotection and relies on the pigment melanin, which is produced by epidermal melanocytes and transferred to surrounding keratinocytes. While the molecular mechanisms of melanin synthesis and transport in melanocytes are now well characterized, much less is known about melanin transfer and processing within keratinocytes. Over the past few decades, distinct models have been proposed to explain how melanin transfer occurs at the cellular and molecular levels. However, this remains a debated topic, as up to four different models have been proposed, with evidence presented supporting each. Here, we review the current knowledge on the regulation of melanin exocytosis, internalization, processing, and polarization. Regarding the different transfer models, we discuss how these might co-exist to regulate skin pigmentation under different conditions, i.e., constitutive and facultative skin pigmentation or physiological and pathological conditions. Moreover, we discuss recent evidence that sheds light on the regulation of melanin exocytosis by melanocytes and internalization by keratinocytes, as well as how melanin is stored within these cells in a compartment that we propose be named the melanokerasome. Finally, we review the state of the art on the molecular mechanisms that lead to melanokerasome positioning above the nuclei of keratinocytes, forming supranuclear caps that shield the nuclear DNA from UV radiation. Thus, we provide a comprehensive overview of the current knowledge on the molecular mechanisms regulating skin pigmentation, from melanin exocytosis by melanocytes and internalization by keratinocytes to processing and polarization within keratinocytes. A better knowledge of these molecular mechanisms will clarify long-lasting questions in the field that are crucial for the understanding of skin pigmentation and can shed light on fundamental aspects of organelle biology. Ultimately, this knowledge can lead to novel therapeutic strategies to treat hypo- or hyper-pigmentation disorders, which have a high socio-economic burden on patients and healthcare systems worldwide, as well as cosmetic applications.
Keywords: keratinocyte; melanin; melanin polarization; melanin processing; melanin transfer; melanocore; melanocyte; melanokerasome; melanosome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

