Real-World Use of Highly Sensitive Liquid Biopsy Monitoring in Metastatic Breast Cancer Patients Treated with Endocrine Agents after Exposure to Aromatase Inhibitors
- PMID: 37511178
- PMCID: PMC10379453
- DOI: 10.3390/ijms241411419
Real-World Use of Highly Sensitive Liquid Biopsy Monitoring in Metastatic Breast Cancer Patients Treated with Endocrine Agents after Exposure to Aromatase Inhibitors
Abstract
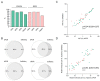
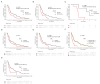
Endocrine-resistant, hormone receptor-positive, and HER2-negative (HR+/HER2-) metastatic breast cancer (mBC) is largely governed by acquired mutations in the estrogen receptor, which promote ligand-independent activation, and by truncal alterations in the PI3K signaling pathway, with a broader range of gene alterations occurring with less prevalence. Circulating tumor DNA (ctDNA)-based technologies are progressively permeating the clinical setting. However, their utility for serial monitoring has been hindered by their significant costs, inter-technique variability, and real-world patient heterogeneity. We interrogated a longitudinal collection of 180 plasma samples from 75 HR+/HER2- mBC patients who progressed or relapsed after exposure to aromatase inhibitors and were subsequently treated with endocrine therapy (ET) by means of highly sensitive and affordable digital PCR and SafeSEQ sequencing. Baseline PIK3CA and TP53 mutations were prognostic of a shorter progression-free survival in our population. Mutant PIK3CA was prognostic in the subset of patients receiving fulvestrant monotherapy after progression to a CDK4/6 inhibitor (CDK4/6i)-containing regimen, and its suppression was predictive in a case of long-term benefit with alpelisib. Mutant ESR1 was prognostic in patients who did not receive concurrent CDK4/6i, an impact influenced by the variant allele frequency, and its early suppression was strongly predictive of efficacy and associated with long-term benefit in the whole cohort. Mutations in ESR1, TP53, and KRAS emerged as putative drivers of acquired resistance. These findings collectively contribute to the characterization of longitudinal ctDNA in real-world cases of HR+/HER2- mBC previously exposed to aromatase inhibitors and support ongoing studies either targeting actionable alterations or leveraging the ultra-sensitive tracking of ctDNA.
Keywords: breast cancer; ctDNA; endocrine resistance; liquid biopsy; precision medicine; real-world evidence.
Conflict of interest statement
JAGS: consultancy for Seagen, Gilead, Sanofi, Novartis, Celgene, Eli Lilly, EISAI, AstraZeneca, Daiichi Sankyo, MSD, and Pierre Fabre; research support (to institution) from AstraZeneca; travel support from Novartis, Roche, and Pfizer. FM: advisory for Roche/Genentech, Novartis, Pfizer, AstraZeneca, MSD-Merck, and Daiichi Sankyo/AstraZeneca; travel support from Roche/Genentech, Pfizer, Novartis, and Daiichi Sankyo/AstraZeneca. DWC: consultancy for AstraZeneca, Exact Sciences, Eisai, Gilead, GlaxoSmithKline, Inivata, Merck, Novartis, Pfizer, and Roche; research support (to institution) from AstraZeneca, Gilead, GlaxoSmithKline, Inivata, Merck, Pfizer, and Roche; and holds intellectual property as co-inventor on a patent (US62/675,228) titled “Methods of treating cancers characterized by a high expression level of spindle and kinetochore associated complex subunit 3 (ska3) gene”. PPS: consultancy for Bristol-Myers Squibb, Merck, MSD. JS and FJ are employees of Sysmex Inostics Inc. The rest of the authors declare no conflicts of interest relevant to the publication of this article. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
Molecular Profiling of Endocrine Resistance in HR+/HER2-Metastatic Breast Cancer: Insights from Extracellular Vesicles-Derived DNA and ctDNA in Liquid Biopsies.Int J Mol Sci. 2024 Dec 4;25(23):13045. doi: 10.3390/ijms252313045. Int J Mol Sci. 2024. PMID: 39684756 Free PMC article.
-
Practical Treatment Strategies and Future Directions After Progression While Receiving CDK4/6 Inhibition and Endocrine Therapy in Advanced HR+/HER2- Breast Cancer.Clin Breast Cancer. 2020 Feb;20(1):1-11. doi: 10.1016/j.clbc.2019.06.017. Epub 2019 Aug 23. Clin Breast Cancer. 2020. PMID: 31780379
-
Effectiveness of Alpelisib + Fulvestrant Compared with Real-World Standard Treatment Among Patients with HR+, HER2-, PIK3CA-Mutated Breast Cancer.Oncologist. 2021 Jul;26(7):e1133-e1142. doi: 10.1002/onco.13804. Epub 2021 May 13. Oncologist. 2021. PMID: 33909934 Free PMC article. Clinical Trial.
-
Everolimus versus alpelisib in advanced hormone receptor-positive HER2-negative breast cancer: targeting different nodes of the PI3K/AKT/mTORC1 pathway with different clinical implications.Breast Cancer Res. 2020 Apr 6;22(1):33. doi: 10.1186/s13058-020-01271-0. Breast Cancer Res. 2020. PMID: 32252811 Free PMC article. Review.
-
Clinical Challenges in the Management of Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: A Literature Review.Adv Ther. 2021 Jan;38(1):109-136. doi: 10.1007/s12325-020-01552-2. Epub 2020 Nov 15. Adv Ther. 2021. PMID: 33190190 Free PMC article. Review.
Cited by
-
Cell-free tumor DNA analysis in advanced or metastatic breast cancer patients: mutation frequencies, testing intention, and clinical impact.Precis Clin Med. 2024 Dec 24;8(1):pbae034. doi: 10.1093/pcmedi/pbae034. eCollection 2025 Mar. Precis Clin Med. 2024. PMID: 39839709 Free PMC article.
-
Development of a prediction model for ctDNA detection (Cir-Predict) in breast cancer.Breast Cancer Res Treat. 2025 Jun;211(2):331-339. doi: 10.1007/s10549-025-07647-0. Epub 2025 Mar 7. Breast Cancer Res Treat. 2025. PMID: 40055250 Free PMC article.
-
Gene expression analysis in circulating tumour cells to determine resistance to CDK4/6 inhibitors plus endocrine therapy in HR + /HER2- metastatic breast cancer patients.J Transl Med. 2025 Apr 4;23(1):400. doi: 10.1186/s12967-025-06374-w. J Transl Med. 2025. PMID: 40186268 Free PMC article.
-
Elacestrant in ER+, HER2- Metastatic Breast Cancer with ESR1-Mutated Tumors: Subgroup Analyses from the Phase III EMERALD Trial by Prior Duration of Endocrine Therapy plus CDK4/6 Inhibitor and in Clinical Subgroups.Clin Cancer Res. 2024 Oct 1;30(19):4299-4309. doi: 10.1158/1078-0432.CCR-24-1073. Clin Cancer Res. 2024. PMID: 39087959 Free PMC article. Clinical Trial.
-
Molecular Profiling of Endocrine Resistance in HR+/HER2-Metastatic Breast Cancer: Insights from Extracellular Vesicles-Derived DNA and ctDNA in Liquid Biopsies.Int J Mol Sci. 2024 Dec 4;25(23):13045. doi: 10.3390/ijms252313045. Int J Mol Sci. 2024. PMID: 39684756 Free PMC article.
References
-
- Pascual J., Attard G., Bidard F.-C., Curigliano G., De Mattos-Arruda L., Diehn M., Italiano A., Lindberg J., Merker J.D., Montagut C., et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: A report from the ESMO Precision Medicine Working Group. Ann Oncol. 2022;33:750–768. doi: 10.1016/j.annonc.2022.05.520. - DOI - PubMed
-
- Turner N.C., Kingston B., Kilburn L.S., Kernaghan S., Wardley A.M., Macpherson I.R., Baird R.D., Roylance R., Stephens P., Oikonomidou O., et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): A multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020;21:1296–1308. doi: 10.1016/S1470-2045(20)30444-7. - DOI - PMC - PubMed
-
- Howell S.J., Casbard A., Carucci M., Ingarfield K., Butler R., Morgan S., Meissner M., Bale C., Bezecny P., Moon S., et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive, HER2-negative breast cancer (FAKTION): Overall survival, updated progression-free survival, and expanded biomarker analysis from a randomised, phase 2 trial. Lancet Oncol. 2022;23:851–864. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

