Vascular Ageing: Mechanisms, Risk Factors, and Treatment Strategies
- PMID: 37511296
- PMCID: PMC10380571
- DOI: 10.3390/ijms241411538
Vascular Ageing: Mechanisms, Risk Factors, and Treatment Strategies
Abstract
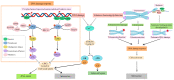
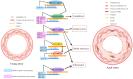
Ageing constitutes the biggest risk factor for poor health and adversely affects the integrity and function of all the cells, tissues, and organs in the human body. Vascular ageing, characterised by vascular stiffness, endothelial dysfunction, increased oxidative stress, chronic low-grade inflammation, and early-stage atherosclerosis, may trigger or exacerbate the development of age-related vascular diseases, which each year contribute to more than 3.8 million deaths in Europe alone and necessitate a better understanding of the mechanisms involved. To this end, a large number of recent preclinical and clinical studies have focused on the exponential accumulation of senescent cells in the vascular system and paid particular attention to the specific roles of senescence-associated secretory phenotype, proteostasis dysfunction, age-mediated modulation of certain microRNA (miRNAs), and the contribution of other major vascular risk factors, notably diabetes, hypertension, or smoking, to vascular ageing in the elderly. The data generated paved the way for the development of various senotherapeutic interventions, ranging from the application of synthetic or natural senolytics and senomorphics to attempt to modify lifestyle, control diet, and restrict calorie intake. However, specific guidelines, considering the severity and characteristics of vascular ageing, need to be established before widespread use of these agents. This review briefly discusses the molecular and cellular mechanisms of vascular ageing and summarises the efficacy of widely studied senotherapeutics in the context of vascular ageing.
Keywords: ageing; natural senotherapeutics; senolytics; senomorphics; vasculature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- World Health Organisation Ageing and Health. [(accessed on 12 February 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health.
-
- World Health Organisation World Health Statistics 2022. [(accessed on 12 February 2023)]. Available online: https://cdn.who.int/media/docs/default-source/gho-documents/world-health...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

