Calcium/P53/Ninjurin 1 Signaling Mediates Plasma Membrane Rupture of Acinar Cells in Severe Acute Pancreatitis
- PMID: 37511311
- PMCID: PMC10380776
- DOI: 10.3390/ijms241411554
Calcium/P53/Ninjurin 1 Signaling Mediates Plasma Membrane Rupture of Acinar Cells in Severe Acute Pancreatitis
Abstract
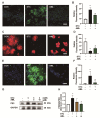
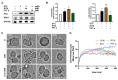
Ninjurin 1 (NINJ1) is a double-transmembrane cell-surface protein that might mediate plasma membrane rupture (PMR) and the diffusion of inflammatory factors. PMR is a characteristic of acinar cell injury in severe acute pancreatitis (SAP). However, the involvement of NINJ1 in mediating the PMR of acinar cells in SAP is currently unclear. Our study has shown that NINJ1 is expressed in acinar cells, and the expression is significantly upregulated in sodium-taurocholate-induced SAP. The knockout of NINJ1 delays PMR in acinar cells and alleviates SAP. Moreover, we observed that NINJ1 expression is mediated by Ca2+ concentration in acinar cells. Importantly, we found that Ca2+ overload drives mitochondrial stress to upregulate the P53/NINJ1 pathway, inducing PMR in acinar cells, and amlodipine, a Ca2+ channel inhibitor, can reduce the occurrence of PMR by decreasing the concentration of Ca2+. Our results demonstrate the mechanism by which NINJ1 induces PMR in SAP acinar cells and provide a potential new target for treatment of SAP.
Keywords: nerve-injury-induced protein 1; plasma membrane rupture; severe acute pancreatitis.
Conflict of interest statement
There are no conflict of interest in this article.
Figures
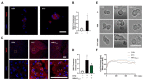
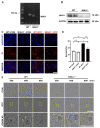
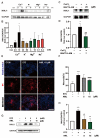
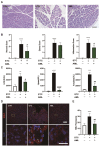
References
-
- Iannuzzi J.P., King J.A., Leong J.H., Quan J., Windsor J.W., Tanyingoh D., Coward S., Forbes N., Heitman S.J., Shaheen A.-A. Global incidence of acute pancreatitis is increasing over time: A systematic review and meta-analysis. Gastroenterology. 2022;162:122–134. doi: 10.1053/j.gastro.2021.09.043. - DOI - PubMed
-
- Xiao A.Y., Tan M.L.Y., Wu L.M., Asrani V.M., Windsor J.A., Yadav D., Petrov M.S. Global incidence and mortality of pancreatic diseases: A systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol. Hepatol. 2016;1:45–55. doi: 10.1016/S2468-1253(16)30004-8. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- ZYXY21002/The 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan Universi
- 161200012/The Innovative Chinese Medicine Preclinical Research Fund of "Liqing No. 2"; West China Hos-pital, Sichuan University
- HXYS19001, HXYS19002/Innovative Chinese Medicine and Health Products Research Academician Workstation of Academician Boli Zhang and Academician Beiwei Zhu, West China Hospital, Sichuan University
- 2022YFS0335/Key R&D project of Science and Technology Department of Sichuan Province
- 2021YFS0408/The Sichuan Province COVID-19 Science and Technology Emergency Project
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

