Effects of Probiotics on Colitis-Induced Exacerbation of Alzheimer's Disease in AppNL-G-F Mice
- PMID: 37511312
- PMCID: PMC10381012
- DOI: 10.3390/ijms241411551
Effects of Probiotics on Colitis-Induced Exacerbation of Alzheimer's Disease in AppNL-G-F Mice
Abstract
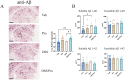
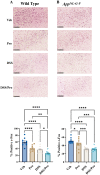
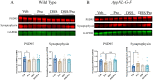
Alzheimer's disease (AD) is characterized by progressive cognitive decline and is a leading cause of death in the United States. Neuroinflammation has been implicated in the progression of AD, and several recent studies suggest that peripheral immune dysfunction may influence the disease. Continuing evidence indicates that intestinal dysbiosis is an attribute of AD, and inflammatory bowel disease (IBD) has been shown to aggravate cognitive impairment. Previously, we separately demonstrated that an IBD-like condition exacerbates AD-related changes in the brains of the AppNL-G-F mouse model of AD, while probiotic intervention has an attenuating effect. In this study, we investigated the combination of a dietary probiotic and an IBD-like condition for effects on the brains of mice. Male C57BL/6 wild type (WT) and AppNL-G-F mice were randomly divided into four groups: vehicle control, oral probiotic, dextran sulfate sodium (DSS), and DSS given with probiotics. As anticipated, probiotic treatment attenuated the DSS-induced colitis disease activity index in WT and AppNL-G-F mice. Although probiotic feeding significantly attenuated the DSS-mediated increase in WT colonic lipocalin levels, it was less protective in the AppNL-G-F DSS-treated group. In parallel with the intestinal changes, combined probiotic and DSS treatment increased microglial, neutrophil elastase, and 5hmC immunoreactivity while decreasing c-Fos staining compared to DSS treatment alone in the brains of WT mice. Although less abundant, probiotic combined with DSS treatment demonstrated a few similar changes in AppNL-G-F brains with increased microglial and decreased c-Fos immunoreactivity in addition to a slight increase in Aβ plaque staining. Both probiotic and DSS treatment also altered the levels of several cytokines in WT and AppNL-G-F brains, with a unique increase in the levels of TNFα and IL-2 being observed in only AppNL-G-F mice following combined DSS and probiotic treatment. Our data indicate that, while dietary probiotic intervention provides protection against the colitis-like condition, it also influences numerous glial, cytokine, and neuronal changes in the brain that may regulate brain function and the progression of AD.
Keywords: Alzheimer’s; amyloid; colitis; intestine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Kumar A., Sidhu J., Goyal A., Tsao J.W. Alzheimer Disease. StatPearls; Treasure Island, FL, USA: 2022.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

