Effective Healing of Staphylococcus aureus-Infected Wounds in Pig Cathelicidin Protegrin-1-Overexpressing Transgenic Mice
- PMID: 37511418
- PMCID: PMC10380341
- DOI: 10.3390/ijms241411658
Effective Healing of Staphylococcus aureus-Infected Wounds in Pig Cathelicidin Protegrin-1-Overexpressing Transgenic Mice
Abstract
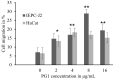
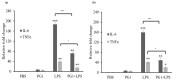
Antimicrobial peptides (AMPs) are promising alternatives to existing treatments for multidrug-resistant bacteria-infected wounds. Therefore, the effect of protegrin-1 (PG1), a potent porcine AMP with broad-spectrum activity, on wound healing was evaluated. PG1-overexpressing transgenic mice were used as an in vivo model to evaluate its healing efficiency against Staphylococcus aureus-infected (106 colony forming units) wounds. We analyzed the wounds under four specific conditions in the presence or absence of antibiotic treatment. We observed the resolution of bacterial infection and formation of neo-epithelium in S. aureus-infected wounds of the mice, even without antibiotic treatment, whereas all wild-type mice with bacterial infection died within 8 to 10 days due to uncontrolled bacterial proliferation. Interestingly, the wound area on day 7 was smaller (p < 0.01) in PG1 transgenic mice than that in the other groups, including antibiotic-treated mice, suggesting that PG1 exerts biological effects other than bactericidal effect. Additionally, we observed that the treatment of primary epidermal keratinocytes with recombinant PG1 enhanced cell migration in in vitro scratch and cell migration assays. This study contributes to the understanding of broad-spectrum endogenous cathelicidins with potent antimicrobial activities, such as PG1, on wound healing. Furthermore, our findings suggest that PG1 is a potent therapeutic candidate for wound healing.
Keywords: antimicrobial peptides; cathelicidin; cell migration; protegrin-1; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

