Evaluation of Novel Enhancer Compounds in Gentamicin-Mediated Readthrough of Nonsense Mutations in Rett Syndrome
- PMID: 37511424
- PMCID: PMC10380790
- DOI: 10.3390/ijms241411665
Evaluation of Novel Enhancer Compounds in Gentamicin-Mediated Readthrough of Nonsense Mutations in Rett Syndrome
Abstract
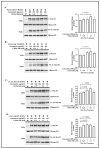
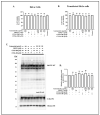
Rett syndrome (RTT), a severe X-linked neurodevelopmental disorder, is primarily caused by mutations in the methyl CpG binding protein 2 gene (MECP2). Over 35% RTT patients carry nonsense mutation in MECP2, making it a suitable candidate disease for nonsense suppression therapy. In our previous study, gentamicin was found to induce readthrough of MECP2 nonsense mutations with modest efficiency. Given the recent discovery of readthrough enhancers, CDX compounds, we herein evaluated the potentiation effect of CDX5-1, CDX5-288, and CDX6-180 on gentamicin-mediated readthrough efficiency in transfected HeLa cell lines bearing the four most common MECP2 nonsense mutations. We showed that all three CDX compounds potentiated gentamicin-mediated readthrough and increased full-length MeCP2 protein levels in cells expressing the R168X, R255X, R270X, and R294X nonsense mutations. Among all three CDX compounds, CDX5-288 was the most potent enhancer and enabled the use of reduced doses of gentamicin, thus mitigating the toxicity. Furthermore, we successfully demonstrated the upregulation of full-length Mecp2 protein expression in fibroblasts derived from Mecp2R255X/Y mice through combinatorial treatment. Taken together, findings demonstrate the feasibility of this combinatorial approach to nonsense suppression therapy for a subset of RTT patients.
Keywords: Rett syndrome; aminoglycosides; methyl-CpG-binding protein; nonsense suppression therapy; readthrough.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Rett A. On a unusual brain atrophy syndrome in hyperammonemia in childhood. Wien. Med. Wochenschr. 1966;116:723–726. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

