Thymocyte Development of Humanized Mice Is Promoted by Interactions with Human-Derived Antigen Presenting Cells upon Immunization
- PMID: 37511462
- PMCID: PMC10380196
- DOI: 10.3390/ijms241411705
Thymocyte Development of Humanized Mice Is Promoted by Interactions with Human-Derived Antigen Presenting Cells upon Immunization
Abstract
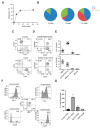
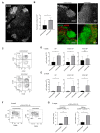
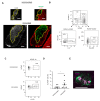
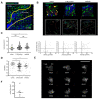
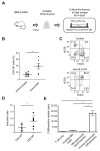
Immune responses in humanized mice are generally inefficient without co-transplantation of human thymus or HLA transgenes. Previously, we generated humanized mice via the intra-bone marrow injection of CD133+ cord blood cells into irradiated adult immunodeficient mice (IBMI-huNSG mice), which could mount functional immune responses against HTLV-1, although the underlying mechanisms were still unknown. Here, we investigated thymocyte development in IBMI-huNSG mice, focusing on the roles of human and mouse MHC restriction. IBMI-huNSG mice had normal developmental profiles but aberrant thymic structures. Surprisingly, the thymic medulla-like regions expanded after immunization due to enhanced thymocyte expansion in association with the increase in HLA-DR+ cells, including CD205+ dendritic cells (DCs). The organ culture of thymus from immunized IBMI-huNSG mice with a neutralizing antibody to HLA-DR showed the HLA-DR-dependent expansion of CD4 single positive thymocytes. Mature peripheral T-cells exhibited alloreactive proliferation when co-cultured with human peripheral blood mononuclear cells. Live imaging of the thymus from immunized IBMI-huNSG mice revealed dynamic adhesive contacts of human-derived thymocytes and DCs accompanied by Rap1 activation. These findings demonstrate that an increase in HLA-DR+ cells by immunization promotes HLA-restricted thymocyte expansion in humanized mice, offering a unique opportunity to generate humanized mice with ease.
Keywords: MHC; dendritic cells; humanized mice; immunization; thymus development.
Conflict of interest statement
The authors declare that they have no competing financial interest.
Figures
Similar articles
-
Dendritic type, accessory cells within the mammalian thymic microenvironment. Antigen presentation in the dendritic neuro-endocrine-immune cellular network.In Vivo. 1997 Jul-Aug;11(4):351-70. In Vivo. 1997. PMID: 9292303
-
T-cell apoptosis and differential human leucocyte antigen class II expression in human thymus.Immunology. 2000 Feb;99(2):249-56. doi: 10.1046/j.1365-2567.2000.00940.x. Immunology. 2000. PMID: 10692044 Free PMC article.
-
Thymocyte LFA-1 and thymic epithelial cell ICAM-1 molecules mediate binding of activated human thymocytes to thymic epithelial cells.J Immunol. 1990 Apr 15;144(8):2931-9. J Immunol. 1990. PMID: 1691223
-
Cytokines in the thymus: production and biological effects.Curr Med Chem. 2004 Feb;11(4):447-64. doi: 10.2174/0929867043455972. Curr Med Chem. 2004. PMID: 14965226 Review.
-
Control of the thymic microenvironment by growth hormone/insulin-like growth factor-I-mediated circuits.Neuroimmunomodulation. 1995 Nov-Dec;2(6):313-8. doi: 10.1159/000097210. Neuroimmunomodulation. 1995. PMID: 8840333 Review.
References
MeSH terms
Substances
Grants and funding
- 20K17036/Japan Society for the Promotion of Science
- a cooperative research grant from the Institute for Enzyme Research Joint Usage/Research Center/Tokushima University
- a "Private University Research Branding Project on intractable immune and allergic diseases" grant from Kansai Medical University/Kansai Medical University
LinkOut - more resources
Full Text Sources
Research Materials

