Genome-Wide Identification of Aqp Family Related to Spermatogenesis in Turbot (Scophthalmus maximus)
- PMID: 37511528
- PMCID: PMC10380888
- DOI: 10.3390/ijms241411770
Genome-Wide Identification of Aqp Family Related to Spermatogenesis in Turbot (Scophthalmus maximus)
Abstract
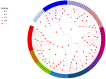
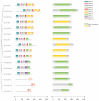
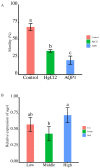
The development and maturation of sperm entails intricate metabolic processes involving water molecules, amino acids, hormones, and various substances. Among these processes, the role of aquaporins (aqps) in the testis is crucial. Turbot (Scophthalmus maximus) is a significant marine flatfish species in China; however, natural egg laying in females is not feasible under cultured conditions. Consequently, artificial insemination becomes necessary, requiring the retrieval of sperm and eggs through artificial methods. In this study, we combined genomic, transcriptomics, RT-qPCR, computer-assisted sperm analysis (CASA), and immunohistochemistry to investigate the involvement of the aqp family in spermatogenesis in turbot. Through genomic data analysis, we identified 16 aqps genes dispersed across 13 chromosomes, each exhibiting the characteristic major intrinsic protein (MIP) domain associated with AQPs. The results from RNA-seq and RT-qPCR analysis revealed prominent expression of aqp4, 10, and 12 during the proliferative stage, whereas aqp1 showed primary expression during the mature stage. aqp11 displayed high expression levels during both MSII and MSV stages, potentially contributing significantly to the proliferation and maturation of male germ cells. Conversely, aqp8 showed elevated expression levels during the MSIII, MSIII-IV, and MSIV stages, suggesting its direct involvement in spermiogenesis. Immunohistochemical analysis unveiled the predominant localization of AQP1 protein in male germ cells rather than Sertoli cells, specifically concentrated in the head of sperm within cysts. Furthermore, a noteworthy decline in sperm motility was observed when sperm were subjected to treatment with either the AQP1-specific inhibitor (HgCl2) or the AQP1 antibody. However, no direct correlation was found between the expression of Smaqp1 and sperm quality. Overall, these findings provide new insights into the involvement of aqps in teleost spermatogenesis. Moreover, they hold potential for improving techniques related to sperm activation and cryopreservation, offering valuable knowledge for future advancements in this field.
Keywords: aqps; sperm; spermatogenesis; turbot.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

