A New Role for Yeast Cells in Health and Nutrition: Antioxidant Power Assessment
- PMID: 37511557
- PMCID: PMC10380906
- DOI: 10.3390/ijms241411800
A New Role for Yeast Cells in Health and Nutrition: Antioxidant Power Assessment
Abstract
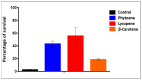
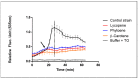
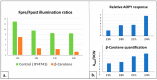
As the use of antioxidant compounds in the domains of health, nutrition and well-being is exponentially rising, there is an urgent need to quantify antioxidant power quickly and easily, ideally within living cells. We developed an Anti Oxidant Power in Yeast (AOPY) assay which allows for the quantitative measurement of the Reactive Oxygen Species (ROS) and free-radical scavenging effects of various molecules in a high-throughput compatible format. Key parameters for Saccharomyces cerevisiae were investigated, and the optimal values were determined for each of them. The cell density in the reaction mixture was fixed at 0.6; the concentration of the fluorescent biosensor (TO) was found to be optimal at 64 µM, and the strongest response was observed for exponentially growing cells. Our optimized procedure allows accurate quantification of the antioxidant effect in yeast of well-known antioxidant molecules: resveratrol, epigallocatechin gallate, quercetin and astaxanthin added in the culture medium. Moreover, using a genetically engineered carotenoid-producing yeast strain, we realized the proof of concept of the usefulness of this new assay to measure the amount of β-carotene directly inside living cells, without the need for cell lysis and purification.
Keywords: LUCS; antioxidant; biosensor; carotenoids; synthetic biology; yeasts.
Conflict of interest statement
C.F. declares being inventor of the patent EP2235505 covering LUCS method. C.F., M.R, and A.F are employees of Anti Oxidant Power—AOP, the start-up company which operates the patent. The other authors declare no conflict of interest.
Figures

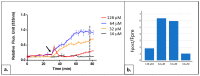
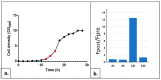
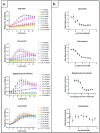
Similar articles
-
Anti-inflammatory Activity of β-Carotene, Lycopene and Tri-n-butylborane, a Scavenger of Reactive Oxygen Species.In Vivo. 2018 Mar-Apr;32(2):255-264. doi: 10.21873/invivo.11232. In Vivo. 2018. PMID: 29475907 Free PMC article.
-
AOP1, a New Live Cell Assay for the Direct and Quantitative Measure of Intracellular Antioxidant Effects.Antioxidants (Basel). 2020 Jun 1;9(6):471. doi: 10.3390/antiox9060471. Antioxidants (Basel). 2020. PMID: 32492957 Free PMC article.
-
In vivo and in vitro evidences that carotenoids could modulate the neutrophil respiratory burst during dietary manipulation.Eur J Nutr. 2005 Mar;44(2):114-20. doi: 10.1007/s00394-004-0501-3. Epub 2004 May 6. Eur J Nutr. 2005. PMID: 15309430
-
[Carotenoids as natural antioxidants].Postepy Hig Med Dosw (Online). 2015 Apr 7;69:418-28. doi: 10.5604/17322693.1148335. Postepy Hig Med Dosw (Online). 2015. PMID: 25897101 Review. Polish.
-
Yeast carotenoids: production and activity as antimicrobial biomolecule.Arch Microbiol. 2021 Apr;203(3):873-888. doi: 10.1007/s00203-020-02111-7. Epub 2020 Nov 5. Arch Microbiol. 2021. PMID: 33151382 Review.
Cited by
-
Anti-Colon Cancer Effects of Lysate from Potential Probiotic Yeast Strains Derived from Human Breast Milk.Probiotics Antimicrob Proteins. 2024 Dec 4. doi: 10.1007/s12602-024-10410-4. Online ahead of print. Probiotics Antimicrob Proteins. 2024. PMID: 39627436
-
In Vivo Antioxidant Activity of Common Dietary Flavonoids: Insights from the Yeast Model Saccharomyces cerevisiae.Antioxidants (Basel). 2024 Sep 12;13(9):1103. doi: 10.3390/antiox13091103. Antioxidants (Basel). 2024. PMID: 39334762 Free PMC article.
References
-
- Dufour C., Villa-Rodriguez J.A., Furger C., Lessard-Lord J., Gironde C., Rigal M., Badr A., Desjardins Y., Guyonnet D. Cellular antioxidant effect of an aronia extract and its polyphenolic fractions enriched in proanthocyanidins, phenolic acids, and anthocyanins. Antioxidants. 2022;11:1561. doi: 10.3390/antiox11081561. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

