CAR-T State of the Art and Future Challenges, A Regulatory Perspective
- PMID: 37511562
- PMCID: PMC10380644
- DOI: 10.3390/ijms241411803
CAR-T State of the Art and Future Challenges, A Regulatory Perspective
Abstract
This review is an outlook on CAR-T development up to the beginning of 2023, with a special focus on the European landscape and its regulatory field, highlighting the main features and limitations affecting this innovative therapy in cancer treatment. We analysed the current state of the art in the EU and set out a showcase of the field's potential advancements in the coming years. For this analysis, the data used came from the available scientific literature as well as from the European Medicines Agency and from clinical trial databases. The latter were investigated to query the studies on CAR-Ts that are active and/or relevant to the review process. As of this writing, CAR-Ts have started to move past the "ceiling" of third-line treatment with positive results in comparison trials with the Standard of Care (SoC). One such example is the trial Zuma-7 (NCT03391466), which resulted in approval of CAR-T products (Yescarta™) for second-line treatment, a crucial achievement for the field which can increase the use of this type of therapy. Despite exciting results in clinical trials, limitations are still many: they regard access, production, duration of response, resistance, safety, overall efficacy, and cost mitigation strategies. Nonetheless, CAR-T constructs are becoming more diverse, and the technology is starting to produce some remarkable results in treating diseases other than cancer.
Keywords: ATMP; CAR-T; EMA; compassionate use; hospital exemption; rare diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
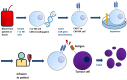
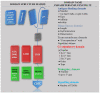
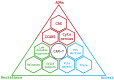

References
-
- Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on Advanced Therapy Medicinal Products and Amending Directive 2001/83/EC and Regulation (EC) No 726/2004 25th of February. [(accessed on 23 May 2023)]. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:324:012....
-
- Regulation (EC) No 141/2000 of the European Parliament and of the Council of 16 December 1999 on Orphan Medicinal Products. [(accessed on 23 May 2023)]. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32000R0141....
-
- European Commission Proposal on Pharmaceutical Legislation. [(accessed on 23 May 2023)]. Available online: https://health.ec.europa.eu/medicinal-products/pharmaceutical-strategy-e....
-
- Compassionate Use of Medicinal Products, Pursuant to Article 83 of Regulation (EC) No 726/2004 Guideline on Compassionate Use of Medicinal Products. [(accessed on 23 May 2023)]. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/g....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

