The Electronic Effects of 3-Methoxycarbonylcoumarin Substituents on Spectral, Antioxidant, and Protein Binding Properties
- PMID: 37511579
- PMCID: PMC10380446
- DOI: 10.3390/ijms241411820
The Electronic Effects of 3-Methoxycarbonylcoumarin Substituents on Spectral, Antioxidant, and Protein Binding Properties
Abstract
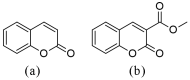
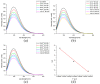
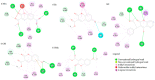
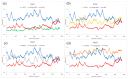
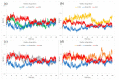
Coumarin derivatives are a class of compounds with pronounced biological activities that depend primarily on the present substituents. Four 3-methoxycarbonylcoumarin derivatives with substituents of different electron-donating/electron-withdrawing abilities (Br, NO2, OH, and OMe) were investigated structurally by NMR, IR, and UV-VIS spectroscopies and density functional theory methods. The appropriate level of theory (B3LYP-D3BJ/6-311++G(d,p) was selected after comparing similar compounds' experimental and theoretical structural parameters. The natural bond orbital and quantum theory of atoms in molecules were employed to investigate the intramolecular interactions governing stability. The electronic effects of substituents mostly affected the aromatic ring that the substituents are directly attached to. The antioxidant properties were investigated by electron paramagnetic resonance spectroscopy towards HO•, and the percentages of reduction were between 13% (6-Br) and 23% (6-OMe). The protein binding properties towards transport proteins were assessed by spectrofluorimetry, molecular docking, and molecular dynamics (MD). The experimentally determined binding energies were well reproduced by molecular docking, showing that the spontaneity of ibuprofen binding was comparable to the investigated compounds. The flexibility of HSA in MD simulations depended on the substituents. These results proved the importance of electronic effects for the protein binding affinities and antioxidant properties of coumarin derivatives.
Keywords: BSA; DFT; EPR; coumarin; molecular dynamics.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Abernethy J.L. The historical and current interest in coumarin. J. Chem. Educ. 1969;46:561. doi: 10.1021/ed046p561. - DOI
-
- Hatano T., Yasuhara T., Fukuda T., Noro T., Okuda T. Phenolic constituents of licorice. II. Structures of licopyranocoumarin, licoarylcoumarin and glisoflavone, and inhibitory effects of licorice phenolics on xanthine oxidase. Chem. Pharm. Bull. 1989;37:3005–3009. doi: 10.1248/cpb.37.3005. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

