Tea Polyphenols Protects Tracheal Epithelial Tight Junctions in Lung during Actinobacillus pleuropneumoniae Infection via Suppressing TLR-4/MAPK/PKC-MLCK Signaling
- PMID: 37511601
- PMCID: PMC10380469
- DOI: 10.3390/ijms241411842
Tea Polyphenols Protects Tracheal Epithelial Tight Junctions in Lung during Actinobacillus pleuropneumoniae Infection via Suppressing TLR-4/MAPK/PKC-MLCK Signaling
Abstract
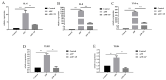
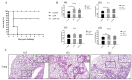
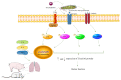
Actinobacillus pleuropneumoniae (APP) is the causative pathogen of porcine pleuropneumonia, a highly contagious respiratory disease in the pig industry. The increasingly severe antimicrobial resistance in APP urgently requires novel antibacterial alternatives for the treatment of APP infection. In this study, we investigated the effect of tea polyphenols (TP) against APP. MIC and MBC of TP showed significant inhibitory effects on bacteria growth and caused cellular damage to APP. Furthermore, TP decreased adherent activity of APP to the newborn pig tracheal epithelial cells (NPTr) and the destruction of the tight adherence junction proteins β-catenin and occludin. Moreover, TP improved the survival rate of APP infected mice but also attenuated the release of the inflammation-related cytokines IL-6, IL-8, and TNF-α. TP inhibited activation of the TLR/MAPK/PKC-MLCK signaling for down-regulated TLR-2, TLR4, p-JNK, p-p38, p-PKC-α, and MLCK in cells triggered by APP. Collectively, our data suggest that TP represents a promising therapeutic agent in the treatment of APP infection.
Keywords: Actinobacillus pleuropneumoniae; TLR-4/MAPK/PKC-MLCK signaling; epithelial barrier; tea polyphenols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Caruana M., Vassallo N. Tea Polyphenols in Parkinson’s Disease. Adv. Exp. Med. Biol. 2015;863:117–137. - PubMed
-
- Zou L.-Q., Liu W., Liu W.-L., Liang R.-H., Li T., Liu C.-M., Cao Y.-L., Niu J., Liu Z. Characterization and Bioavailability of Tea Polyphenol Nanoliposome Prepared by Combining an Ethanol Injection Method with Dynamic High-Pressure Microfluidization. J. Agric. Food Chem. 2014;62:934–941. doi: 10.1021/jf402886s. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

