mTOR Inhibition via Low-Dose, Pulsed Rapamycin with Intraovarian Condensed Platelet Cytokines: An Individualized Protocol to Recover Diminished Reserve?
- PMID: 37511761
- PMCID: PMC10381109
- DOI: 10.3390/jpm13071147
mTOR Inhibition via Low-Dose, Pulsed Rapamycin with Intraovarian Condensed Platelet Cytokines: An Individualized Protocol to Recover Diminished Reserve?
Abstract
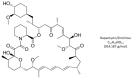
No major breakthroughs have entered mainstream clinical fertility practice since egg donation and intracytoplasmic sperm injection decades ago, and oocyte deficits secondary to advanced age continue as the main manifestation of diminished ovarian reserve. In the meantime, several unproven IVF 'accessories' have emerged including so-called ovarian rejuvenation which entails placing fresh autologous platelet-rich plasma (PRP) directly into ovarian tissue. Among cellular responses attributed to this intervention are reduced oxidative stress, slowed apoptosis and improved metabolism. Besides having an impact on the existing follicle pool, platelet growth factors might also facilitate de novo oocyte recruitment by specified gene upregulation targeting uncommitted ovarian stem cells. Given that disordered activity at the mechanistic target of rapamycin (mTOR) has been shown to exacerbate or accelerate ovarian aging, PRP-discharged plasma cytokines combined with mTOR suppression by pulsed/cyclic rapamycin represents a novel fusion technique to enhance ovarian function. While beneficial effects have already been observed experimentally in oocytes and embryos with mTOR inhibition alone, this proposal is the first to discuss intraovarian platelet cytokines followed by low-dose, phased rapamycin. For refractory cases, this investigational, tailored approach could amplify or sustain ovarian capacity sufficient to permit retrieval of competent oocytes via distinct but complementary pathways-thus reducing dependency on oocyte donation.
Keywords: IVF; PRP; mTOR; ovarian reserve; platelet cytokines; rapamycin.
Conflict of interest statement
ESS has been awarded U.S. Trademark #88505430 for process and method using autologous platelet cytokines for ovarian therapy. CH, SHW and SLT have no disclosures.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

