Phosphorylation Mimetic of Myosin Regulatory Light Chain Mitigates Cardiomyopathy-Induced Myofilament Impairment in Mouse Models of RCM and DCM
- PMID: 37511838
- PMCID: PMC10381296
- DOI: 10.3390/life13071463
Phosphorylation Mimetic of Myosin Regulatory Light Chain Mitigates Cardiomyopathy-Induced Myofilament Impairment in Mouse Models of RCM and DCM
Abstract
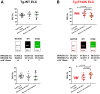
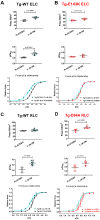
This study focuses on mimicking constitutive phosphorylation in the N-terminus of the myosin regulatory light chain (S15D-RLC) as a rescue strategy for mutation-induced cardiac dysfunction in transgenic (Tg) models of restrictive (RCM) and dilated (DCM) cardiomyopathy caused by mutations in essential (ELC, MYL3 gene) or regulatory (RLC, MYL2 gene) light chains of myosin. Phosphomimetic S15D-RLC was reconstituted in left ventricular papillary muscle (LVPM) fibers from two mouse models of cardiomyopathy, RCM-E143K ELC and DCM-D94A RLC, along with their corresponding Tg-ELC and Tg-RLC wild-type (WT) mice. The beneficial effects of S15D-RLC in rescuing cardiac function were manifested by the S15D-RLC-induced destabilization of the super-relaxed (SRX) state that was observed in both models of cardiomyopathy. S15D-RLC promoted a shift from the SRX state to the disordered relaxed (DRX) state, increasing the number of heads readily available to interact with actin and produce force. Additionally, S15D-RLC reconstituted with fibers demonstrated significantly higher maximal isometric force per cross-section of muscle compared with reconstitution with WT-RLC protein. The effects of the phosphomimetic S15D-RLC were compared with those observed for Omecamtiv Mecarbil (OM), a myosin activator shown to bind to the catalytic site of cardiac myosin and increase myocardial contractility. A similar SRX↔DRX equilibrium shift was observed in OM-treated fibers as in S15D-RLC-reconstituted preparations. Additionally, treatment with OM resulted in significantly higher maximal pCa 4 force per cross-section of muscle fibers in both cardiomyopathy models. Our results suggest that both treatments with S15D-RLC and OM may improve the function of myosin motors and cardiac muscle contraction in RCM-ELC and DCM-RLC mice.
Keywords: N-terminal protein modification; S15D-RLC phosphomimetic; Tg mice; cardiomyopathy; myosin ELC; myosin RLC; super-relaxed state (SRX).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Geeves M.A., Holmes K.C. The molecular mechanism of muscle contraction. Adv. Protein Chem. 2005;71:161–193. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

