Compound K Production: Achievements and Perspectives
- PMID: 37511939
- PMCID: PMC10381408
- DOI: 10.3390/life13071565
Compound K Production: Achievements and Perspectives
Abstract
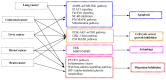
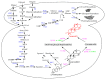
Compound K (CK) is one of the major metabolites found in mammalian blood and organs following oral administration of Panax plants. CK, also known as minor ginsenoside, can be absorbed in the systemic circulation. It has garnered significant attention in healthcare and medical products due to its pharmacological activities, such as antioxidation, anticancer, antiproliferation, antidiabetics, neuroprotection, and anti-atherogenic activities. However, CK is not found in natural ginseng plants but in traditional chemical synthesis, which uses toxic solvents and leads to environmental pollution during the harvest process. Moreover, enzymatic reactions are impractical for industrial CK production due to low yield and high costs. Although CK could be generated from major ginsenosides, most ginsenosides, including protopanaxatriol-oleanane and ocotillol-type, are not converted into CK by catalyzing β-glucosidase. Therefore, microbial cell systems have been used as a promising solution, providing a safe and efficient approach to CK production. This review provides a summary of various approaches for the production of CK, including chemical and enzymatic reactions, biotransformation by the human intestinal bacteria and endophytes as well as engineered microbes. Moreover, the approaches for CK production have been discussed to improve the productivity of target compounds.
Keywords: Compound K (CK); Panax ginseng; Saccharomyces cerevisiae; endophytes; β-glucosidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

