Evaluation of Cytotoxicity of Hyaluronic Acid/Chitosan/Bacterial Cellulose-Based Membrane
- PMID: 37512462
- PMCID: PMC10383227
- DOI: 10.3390/ma16145189
Evaluation of Cytotoxicity of Hyaluronic Acid/Chitosan/Bacterial Cellulose-Based Membrane
Abstract
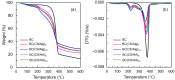
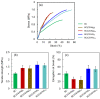
Novel wound dressing materials are required to non-cytotoxic with a viable cell ratio of above 92%. Herein, the cytotoxicity of hyaluronic acid/chitosan/bacterial cellulose-based (BC(CS/HA)) membranes are evaluated and compared to that of alginate/chitosan/bacterial cellulose-based (BC(CS/Alg)) membranes was investigated. Multilayer membranes with up to ten CS/HA or CS/Alg layers were prepared using the layer-by-layer (LBL) method. Scanning electron microscopy showed that the diameters of the fibers in the BC(CS/Alg) and BC(CS/HA) membranes were larger than those in a BC membrane. The cytotoxicity was analyzed using BALB-3T3 clone A31 cells (mouse fibroblasts, 1 × 104 cells/well). The BC(CS/HA)5 and BC(CS/HA)10 membranes exhibited high biocompatibility, with the cell viabilities of 94% and 87% at 5 d, respectively, compared to just 82% for the BC(CS/Alg)5 and BC(CS/Alg)10 membranes with same numbers of layers. These results suggested that BC(CS/HA)5 is a promising material for wound dressings.
Keywords: alginate; layer by layer; wound dressing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Chitosan/polyanion surface modification of styrene-butadiene-styrene block copolymer membrane for wound dressing.Mater Sci Eng C Mater Biol Appl. 2014 Jan 1;34:140-8. doi: 10.1016/j.msec.2013.09.001. Epub 2013 Sep 11. Mater Sci Eng C Mater Biol Appl. 2014. PMID: 24268243
-
Thermal Properties and Structural Features of Multilayer Films Based on Chitosan and Anionic Polysaccharides.Biomolecules. 2021 May 19;11(5):762. doi: 10.3390/biom11050762. Biomolecules. 2021. PMID: 34069622 Free PMC article.
-
Bacterial Cellulose (Komagataeibacter rhaeticus) Biocomposites and Their Cytocompatibility.Materials (Basel). 2020 Oct 14;13(20):4558. doi: 10.3390/ma13204558. Materials (Basel). 2020. PMID: 33066426 Free PMC article.
-
Bacterial Cellulose-Based Materials as Dressings for Wound Healing.Pharmaceutics. 2023 Jan 27;15(2):424. doi: 10.3390/pharmaceutics15020424. Pharmaceutics. 2023. PMID: 36839745 Free PMC article. Review.
-
Overview of bacterial cellulose composites: a multipurpose advanced material.Carbohydr Polym. 2013 Nov 6;98(2):1585-98. doi: 10.1016/j.carbpol.2013.08.018. Epub 2013 Aug 15. Carbohydr Polym. 2013. PMID: 24053844 Review.
Cited by
-
Production, Optimization, and Characterization of Bio-cellulose Produced from Komagataeibacter (Acetobacter aceti MTCC 3347) Usage of Food Sources as Media.Recent Adv Food Nutr Agric. 2024;15(3):215-227. doi: 10.2174/012772574X284979231231102050. Recent Adv Food Nutr Agric. 2024. PMID: 38305312
References
-
- Husteden C., Doberenz F., Goergen N., Pinnapireddy S.R., Janich C., Langner A., Syrowatka F., Repanas A., Erdmann F., Jedelská J., et al. Contact-triggered lipofection from multilayer films designed as surfaces for in situ transfection strategies in tissue engineering. ACS Appl. Mater. Interfaces. 2020;12:8963–8977. doi: 10.1021/acsami.9b18968. - DOI - PubMed
-
- Panda P.K., Yang J.-M., Chang Y.-H. Preparation and characterization of ferulic acid-modified water soluble chitosan and poly (gamma-glutamic acid) polyelectrolyte films through layer-by-layer assembly towards protein adsorption. Int. J. Biol. Macromol. 2021;171:457–464. doi: 10.1016/j.ijbiomac.2020.12.226. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

