Panapophenanthrin, a Rare Oligocyclic Diterpene from Panus strigellus
- PMID: 37512554
- PMCID: PMC10385786
- DOI: 10.3390/metabo13070848
Panapophenanthrin, a Rare Oligocyclic Diterpene from Panus strigellus
Abstract
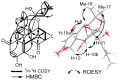
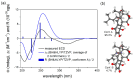
During the course of our search for biologically active secondary metabolites from fungal cultures, a new oligocyclic diterpenoidal derivative, panapophenanthrin (1), was isolated from Panus strigellus. In addition, two known metabolites, panepophenanthrin (2) and dihydrohypnophilin (3), were also obtained. The chemical structures of the isolated compounds were elucidated based on extensive 1D and 2D NMR spectral analyses together with high-resolution electrospray ionization mass spectrometry (HR-ESI-MS). The absolute configuration was determined through TDDFT-ECD calculations. All of the compounds were assessed for their antimicrobial and cytotoxic activities. Compounds 1 and 3 showed moderate to weak activities in the performed antimicrobial assays, while compound 1 exhibited potent cytotoxic activity against the mammalian cell lines mouse fibroblast (L929) and human endocervical adenocarcinoma (KB3.1).
Keywords: antimicrobial; basidiomycota; cytotoxicity; secondary metabolites; white rot fungi.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Chen L., Zhang X., Zhang M., Zhu Y., Zhuo R. Removal of heavy-metal pollutants by white rot fungi: Mechanisms, achievements, and perspectives. J. Clean Prod. 2022;354:131681. doi: 10.1016/j.jclepro.2022.131681. - DOI
-
- Jaén-Gil A., Castellet-Rovira F., Llorca M., Villagrasa M., Sarrà M., Rodríguez-Mozaz S., Barceló D. Fungal treatment of metoprolol and its recalcitrant metabolite metoprolol acid in hospital wastewater: Biotransformation, sorption and ecotoxicological impact. Water Res. 2019;152:171–180. doi: 10.1016/j.watres.2018.12.054. - DOI - PubMed
Grants and funding
- 57552340/German Academic Exchange Service
- ID490821847/Deutsche Forschungsgemeinschaft
- Ref 3.4-1222288-EGY-GF-E/Alexander von Humboldt Foundation
- 80740-056/Ministerio de Ciencia Tecnología e Innovación, Colombia
- 126-13/05/2016/Ministerio de Ambiente y Desarrollo Sostenible de la Republica de Colombia
LinkOut - more resources
Full Text Sources

