Characterization of trans-3-Methylglutaconyl CoA-Dependent Protein Acylation
- PMID: 37512569
- PMCID: PMC10386559
- DOI: 10.3390/metabo13070862
Characterization of trans-3-Methylglutaconyl CoA-Dependent Protein Acylation
Abstract
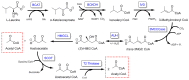
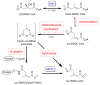
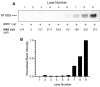
3-methylglutaconyl (3MGC) CoA hydratase (AUH) is the leucine catabolism pathway enzyme that catalyzes the hydration of trans-3MGC CoA to 3-hydroxy, 3-methylglutaryl (HMG) CoA. In several inborn errors of metabolism (IEM), however, metabolic dysfunction can drive this reaction in the opposite direction (the dehydration of HMG CoA). The recent discovery that trans-3MGC CoA is inherently unstable and prone to a series of non-enzymatic chemical reactions provides an explanation for 3MGC aciduria observed in these IEMs. Under physiological conditions, trans-3MGC CoA can isomerize to cis-3MGC CoA, which is structurally poised to undergo intramolecular cyclization with the loss of CoA, generating cis-3MGC anhydride. The anhydride is reactive and has two potential fates; (a) hydrolysis to yield cis-3MGC acid or (b) a reaction with lysine side-chain amino groups to 3MGCylate substrate proteins. An antibody elicited against a 3MGC hapten was employed to investigate protein acylation in incubations containing recombinant AUH, HMG CoA, and bovine serum albumin (BSA). The data obtained show that, as AUH dehydrates HMG CoA to trans-3MGC CoA, BSA is acylated. Moreover, α-3MGC IgG immunoblot signal intensity correlates with AUH concentration, HMG CoA substrate concentration, and incubation time. Thus, protein 3MGCylation may contribute to the phenotypic features associated with IEMs that manifest 3MGC aciduria.
Keywords: 3MGC aciduria; acetyl CoA diversion; immunoblot; inborn error of metabolism; protein 3MGCylation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Wortmann S.B., Duran M., Anikster Y., Barth P.G., Sperl W., Zschocke J., Morava E., Wevers R.A. Inborn errors of metabolism with 3-methylglutaconic aciduria as discriminative feature: Proper classification and nomenclature. J. Inherit. Metab. Dis. 2013;36:923–928. doi: 10.1007/s10545-012-9580-0. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

