A Modified-Herringbone Micromixer for Assessing Zebrafish Sperm (MAGS)
- PMID: 37512621
- PMCID: PMC10386169
- DOI: 10.3390/mi14071310
A Modified-Herringbone Micromixer for Assessing Zebrafish Sperm (MAGS)
Abstract
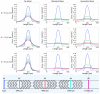
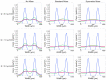
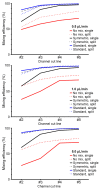
Sperm motility analysis of aquatic model species is important yet challenging due to the small sample volume, the necessity to activate with water, and the short duration of motility. To achieve standardization of sperm activation, microfluidic mixers have shown improved reproducibility over activation by hand, but challenges remain in optimizing and simplifying the use of these microdevices for greater adoption. The device described herein incorporates a novel micromixer geometry that aligns two sperm inlet streams with modified herringbone structures that split and recombine the sample at a 1:6 dilution with water to achieve rapid and consistent initiation of motility. The polydimethylsiloxane (PDMS) chip can be operated in a positive or negative pressure configuration, allowing a simple micropipettor to draw samples into the chip and rapidly stop the flow. The device was optimized to not only activate zebrafish sperm but also enables practical use with standard computer-assisted sperm analysis (CASA) systems. The micromixer geometry could be modified for other aquatic species with differing cell sizes and adopted for an open hardware approach using 3D resin printing where users could revise, fabricate, and share designs to improve standardization and reproducibility across laboratories and repositories.
Keywords: PDMS; high-dilution; microfabricated; micromixer; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Fauvel C., Suquet M., Cosson J. Evaluation of fish sperm quality. J. Appl. Ichthyol. 2010;26:636–643. doi: 10.1111/j.1439-0426.2010.01529.x. - DOI
-
- Chan S.Y., Wang C., Song B.L., Lo T., Leung A., Tsoi W.L., Leung J. Computer-assisted image analysis of sperm concentration in human semen before and after swim-up separation: Comparison with assessment by haemocytometer. Int. J. Androl. 1989;12:339–345. doi: 10.1111/j.1365-2605.1989.tb01322.x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

