Super-Macroporous Pulluan Cryogels as Controlled Active Delivery Systems with Controlled Degradability
- PMID: 37512634
- PMCID: PMC10385955
- DOI: 10.3390/mi14071323
Super-Macroporous Pulluan Cryogels as Controlled Active Delivery Systems with Controlled Degradability
Abstract
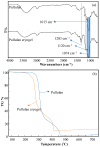
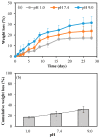
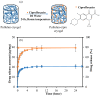
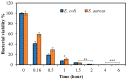
Here, super-macroporous cryogel from a natural polysaccharide, pullulan was synthesized using a cryo-crosslinking technique with divinyl sulfone (DVS) as a crosslinker. The hydrolytic degradation of the pullulan cryogel in various simulated body fluids (pH 1.0, 7.4, and 9.0 buffer solutions) was evaluated. It was observed that the pullulan cryogel degradation was much faster in the pH 9 buffer solution than the pH 1.0 and 7.4 buffer solutions in the same time period. The weight loss of the pullulan cryogel at pH 9.0 within 28 days was determined as 31% ± 2%. To demonstrate the controllable drug delivery potential of pullulan cryogels via degradation, an antibiotic, ciprofloxacin, was loaded into pullulan cryogels (pullulan-cipro), and the loading amount of drug was calculated as 105.40 ± 2.6 µg/mg. The release of ciprofloxacin from the pullulan-cipro cryogel was investigated in vitro at 37.5 °C in physiological conditions (pH 7.4). The amount of drug released within 24 h was determined as 39.26 ± 3.78 µg/mg, which is equal to 41.38% ± 3.58% of the loaded drug. Only 0.1 mg of pullulan-cipro cryogel was found to inhibit half of the growing Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) colonies for 10 min and totally eradicated within 2 h by the release of the loaded antibiotic. No significant toxicity was determined on L929 fibroblast cells for 0.1 mg drug-loaded pullulan cryogel. In contrast, even 1 mg of drug-loaded pullulan cryogel revealed slight toxicity (e.g., 66% ± 9% cell viability) because of the high concentration of released drug.
Keywords: biocompatible/degradable polysaccharide cryogel; ciprofloxacin release; natural polymeric cryogel drug carrier; pullulan cryogel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Akash M.S.H., Rehman K., Chen S. Natural and Synthetic Polymers as Drug Carriers for Delivery of Therapeutic Proteins. Polym. Rev. 2015;55:371–406. doi: 10.1080/15583724.2014.995806. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

