Bacillus subtilis PM5 from Camel Milk Boosts Chicken Immunity and Abrogates Salmonella entertitidis Infections
- PMID: 37512891
- PMCID: PMC10385966
- DOI: 10.3390/microorganisms11071719
Bacillus subtilis PM5 from Camel Milk Boosts Chicken Immunity and Abrogates Salmonella entertitidis Infections
Abstract
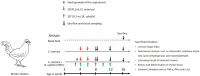
With the practice of a successful livestock industry using antibiotics, which has continued for more than five decades, researchers have long been interested in finding alternatives to antibiotics for poultry production. Probiotics can potentially reduce enteric diseases in livestock and enhance their productivity. The aim of this study was to isolate putative probiotics from camel milk and test them against Salmonella infection as well as host immune development. Thirteen different isolates were obtained from six different camel milk samples from dairy farms in Saudi Arabia. Three of the six isolates (PM1, PM2, PM3, PM4, PM5, and PM6) that showed Gram-positive characters reacted negatively to catalase and hemolytic assays. PM1, PM5, and PM6 showed significant nonpolar surface properties (>51% hydrophobic) and potent antimicrobial activities against avian pathogens, namely S. enterica, S. typhi, S. aureus, and E. coli. PM5 exhibited substantial probiotic traits; therefore, further focus was given to it. PM5 was identified as Bacillus subtilis OQ913924 by the 16S rRNA sequencing method and showed similarity matrix > 99%. An in vivo chicken model was used to access the health benefits of probiotics. After salmonella infection, the mucosal immune response was significantly increased (p < 0.01), and none of the challenge protocols caused mortality or clinical symptoms after infection in intestinal contents. S. enterica organ infiltration in the spleen, thymus, and small intestine was significantly reduced in the B. subtilis PM5-fed chickens. The S. enterica load in chicken feces was reduced from CFU 7.2 to 5.2 in oral-fed B. subtilis PM5-fed chickens. Probiotic-fed chickens showed buffered intestinal content and positively regulated the level of butyric acid (p < 0.05), and intestinal interleukin 1 beta (IL1-β), C-reactive protein (CRP), and interferon gamma (IFN-γ) levels were reduced (p < 0.05). In addition, B. subtilis PM5 showed significant binding to peritoneal macrophages cells and inhibited S. enterica surface adhesion, indicating co-aggregation of B. subtilis PM5 in macrophage cells. It could be concluded that supplementation with probiotics can improve the growth performance of broilers and the quality of broiler chickens against enteric pathogens. The introduction of this probiotic into the commercial poultry feed market in the near future may assist in narrowing the gap that now exists between chicken breeding and consumer demand.
Keywords: Bacillus; camel milk; chicken; infection; probiotics.
Conflict of interest statement
The author declares no conflict of interest.
Figures




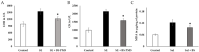


References
-
- Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. - DOI - PubMed
-
- Moussaid S., El Alaoui M.A., Ounine K., Benali A., Bouhlal O., Rkhaila A., Hami H., El Maadoudi E.H. In-vitro evaluation of the probiotic potential and the fermentation profile of Pediococcus and Enterococcus strains isolated from Moroccan camel milk. Arch. Microbiol. 2023;205:1–16. doi: 10.1007/s00203-023-03489-w. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

