Gut Microbiota and Critical Metabolites: Potential Target in Preventing Gestational Diabetes Mellitus?
- PMID: 37512897
- PMCID: PMC10385493
- DOI: 10.3390/microorganisms11071725
Gut Microbiota and Critical Metabolites: Potential Target in Preventing Gestational Diabetes Mellitus?
Abstract
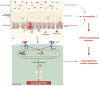
Gestational diabetes mellitus (GDM) is an intractable issue that negatively impacts the quality of pregnancy. The incidence of GDM is on the rise, becoming a major health burden for both mothers and children. However, the specific etiology and pathophysiology of GDM remain unknown. Recently, the importance of gut microbiota and related metabolic molecules has gained prominence. Studies have indicated that women with GDM have significantly distinct gut microbiota and gut metabolites than healthy pregnant women. Given that the metabolic pathways of gut flora and related metabolites have a substantial impact on inflammation, insulin signaling, glucose, and lipid metabolism, and so on, gut microbiota or its metabolites, such as short-chain fatty acids, may play a significant role in both pathogenesis and progression of GDM. Whereas the role of intestinal flora during pregnancy is still in its infancy, this review aims to summarize the effects and mechanisms of gut microbiota and related metabolic molecules involved in GDM, thus providing potential intervention targets.
Keywords: gestational diabetes mellitus; gut microbiota; gut microecology; interaction; short-chain fatty acids.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Sacks D.A., Hadden D.R., Maresh M., Deerochanawong C., Dyer A.R., Metzger B.E., Lowe L.P., Coustan D.R., Hod M., Oats J.J., et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care. 2012;35:526–528. doi: 10.2337/dc11-1641. - DOI - PMC - PubMed
-
- Catalano P.M., McIntyre H.D., Cruickshank J.K., McCance D.R., Dyer A.R., Metzger B.E., Lowe L.P., Trimble E.R., Coustan D.R., Hadden D.R., et al. The hyperglycemia and adverse pregnancy outcome study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35:780–786. doi: 10.2337/dc11-1790. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

