Studies Using Mutant Strains of Azospirillum brasilense Reveal That Atmospheric Nitrogen Fixation and Auxin Production Are Light Dependent Processes
- PMID: 37512900
- PMCID: PMC10383956
- DOI: 10.3390/microorganisms11071727
Studies Using Mutant Strains of Azospirillum brasilense Reveal That Atmospheric Nitrogen Fixation and Auxin Production Are Light Dependent Processes
Abstract
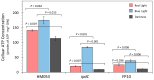
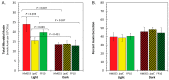
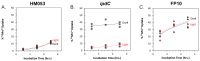
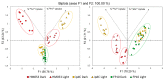
As the use of microbial inoculants in agriculture rises, it becomes important to understand how the environment may influence microbial ability to promote plant growth. This work examines whether there are light dependencies in the biological functions of Azospirillum brasilense, a commercialized prolific grass-root colonizer. Though classically defined as non-phototrophic, A. brasilense possesses photoreceptors that could perceive light conducted through its host's roots. Here, we examined the light dependency of atmospheric biological nitrogen fixation (BNF) and auxin biosynthesis along with supporting processes including ATP biosynthesis, and iron and manganese uptake. Functional mutants of A. brasilense were studied in light and dark environments: HM053 (high BNF and auxin production), ipdC (capable of BNF, deficient in auxin production), and FP10 (capable of auxin production, deficient in BNF). HM053 exhibited the highest rate of nitrogenase activity with the greatest light dependency comparing iterations in light and dark environments. The ipdC mutant showed similar behavior with relatively lower nitrogenase activity observed, while FP10 did not show a light dependency. Auxin biosynthesis showed strong light dependencies in HM053 and FP10 strains, but not for ipdC. Ferrous iron is involved in BNF, and a light dependency was observed for microbial 59Fe2+ uptake in HM053 and ipdC, but not FP10. Surprisingly, a light dependency for 52Mn2+ uptake was only observed in ipdC. Finally, ATP biosynthesis was sensitive to light across all three mutants favoring blue light over red light compared to darkness with observed ATP levels in descending order for HM053 > ipdC > FP10.
Keywords: ATP biosynthesis; Azospirillum brasilense; biological nitrogen fixation; light stimulation; microbial auxin biosynthesis; micronutrient uptake.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Azospirillum brasilense Bacteria Promotes Mn2+ Uptake in Maize with Benefits to Leaf Photosynthesis.Microorganisms. 2022 Jun 25;10(7):1290. doi: 10.3390/microorganisms10071290. Microorganisms. 2022. PMID: 35889009 Free PMC article.
-
Plant-Growth-Promoting Bacteria Can Impact Zinc Uptake in Zea mays: An Examination of the Mechanisms of Action Using Functional Mutants of Azospirillum brasilense.Microorganisms. 2021 May 6;9(5):1002. doi: 10.3390/microorganisms9051002. Microorganisms. 2021. PMID: 34066521 Free PMC article.
-
Functional mutants of Azospirillum brasilense elicit beneficial physiological and metabolic responses in Zea mays contributing to increased host iron assimilation.ISME J. 2021 May;15(5):1505-1522. doi: 10.1038/s41396-020-00866-x. Epub 2021 Jan 6. ISME J. 2021. PMID: 33408368 Free PMC article.
-
Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects.FEMS Microbiol Rev. 2000 Oct;24(4):487-506. doi: 10.1111/j.1574-6976.2000.tb00552.x. FEMS Microbiol Rev. 2000. PMID: 10978548 Review.
-
Key physiological properties contributing to rhizosphere adaptation and plant growth promotion abilities of Azospirillum brasilense.FEMS Microbiol Lett. 2012 Jan;326(2):99-108. doi: 10.1111/j.1574-6968.2011.02407.x. Epub 2011 Oct 3. FEMS Microbiol Lett. 2012. PMID: 22092983 Review.
Cited by
-
Indole-3-acetic acid (IAA) protects Azospirillum brasilense from indole-induced stress.Appl Environ Microbiol. 2025 Apr 23;91(4):e0238424. doi: 10.1128/aem.02384-24. Epub 2025 Mar 25. Appl Environ Microbiol. 2025. PMID: 40130845 Free PMC article.
-
The Hidden World within Plants 2.0.Microorganisms. 2023 Dec 1;11(12):2903. doi: 10.3390/microorganisms11122903. Microorganisms. 2023. PMID: 38138046 Free PMC article.
-
A Study of the Different Strains of the Genus Azospirillum spp. on Increasing Productivity and Stress Resilience in Plants.Plants (Basel). 2025 Jan 18;14(2):267. doi: 10.3390/plants14020267. Plants (Basel). 2025. PMID: 39861620 Free PMC article. Review.
-
Ochrobactrum sp. NBRISH6 Inoculation Enhances Zea mays Productivity, Mitigating Soil Alkalinity and Plant Immune Response.Curr Microbiol. 2023 Aug 25;80(10):328. doi: 10.1007/s00284-023-03441-7. Curr Microbiol. 2023. PMID: 37620623
References
-
- Kumar S., Kateriya S., Singh V.S., Tanwar M., Agarwal S., Singh H., Khurana J.P., Amla D.V., Tripathi A.K. Bacteriophytochrome controls carotenoid-independent response to photodynamic stress in a non-photosynthetic rhizobacterium, Azospirillum brasilense Sp7. Sci. Rep. 2012;2:872. doi: 10.1038/srep00872. - DOI - PMC - PubMed
-
- Kakuszi A., Sárvári É., Solti Á., Czégény G., Hideg É., Hunyadi-Gulyás É., Bóka K., Böddi B. Light piping driven photosynthesis in the soil: Low-light adapted active photosynthetic apparatus in the under-soil hypocotyl segments of bean (Phaseolus vulgaris) J. Photochem. Photobiol. B Biol. 2016;161:422–429. doi: 10.1016/j.jphotobiol.2016.06.009. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

