The Role of Osmolytes and Membrane Lipids in the Adaptation of Acidophilic Fungi
- PMID: 37512905
- PMCID: PMC10383115
- DOI: 10.3390/microorganisms11071733
The Role of Osmolytes and Membrane Lipids in the Adaptation of Acidophilic Fungi
Abstract
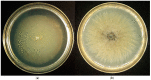
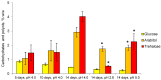
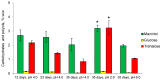
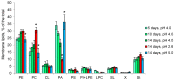
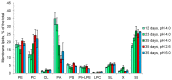
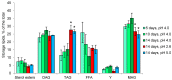
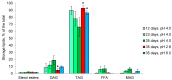
Acidophiles maintain near-neutral intracellular pH using proton pumps. We have suggested the protective role of osmolytes and membrane lipids in the adaptation to an acidic environment. Previously we have observed, for the first time, high levels of trehalose in acidophilic basidiomycete Sistotrema brinkmannii. Here, we have studied the composition of both osmolytes and membrane lipids of two more acidophilic fungi. Trehalose and polyols were among the main osmolytes during growth under optimal conditions (pH 4.0) in basidiomycete Phlebiopsis gigantea and ascomycete Mollisia sp. Phosphatidic acids, phosphatidylethanolamines, phosphatidylcholines, and sterols, were predominant membrane lipids in both fungi. P. gigantea had a narrow optimum of growth at pH 4.0, resulting in a sharp decline of growth rate at pH 2.6 and 5.0, accompanied by a decrease in the number of osmolytes and significant changes in the composition of membrane lipids. In contrast, Mollisia sp. had a broad optimal growth range (pH 3.0-5.0), and the number of osmolytes either stayed the same (at pH 6.0) or increased (at pH 2.6), while membrane lipids composition remained unchanged. Thus, the data obtained indicate the participation of osmolytes and membrane lipids in the adaptation of acidophilic fungi.
Keywords: Mollisia; Phlebiopsis gigantea; extremophilic fungi; phosphatidic acids; trehalose.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the study’s design; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Gross S., Robbins E.I. Acidophilic and Acid-Tolerant Fungi and Yeasts. Hydrobiologia. 2000;433:91–109. doi: 10.1023/A:1004014603333. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

