Clostridioides difficile in Pigs and Dairy Cattle in Northern Italy: Prevalence, Characterization and Comparison between Animal and Human Strains
- PMID: 37512910
- PMCID: PMC10383565
- DOI: 10.3390/microorganisms11071738
Clostridioides difficile in Pigs and Dairy Cattle in Northern Italy: Prevalence, Characterization and Comparison between Animal and Human Strains
Abstract
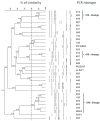
It has been observed that novel strains of Clostridioides difficile can rapidly emerge and move between animal and human hosts. The aim of this study was to investigate the prevalence of C. difficile in pigs and dairy cattle in northern Italy and to characterize and compare C. difficile animal strains with those from patients from the same geographical area. The C. difficile strains were isolated from animals from farms and slaughterhouses (cross-sectional studies) and from neonatal animals with enteric disorders in routine diagnostic investigations (passive surveillance). Samples positive for C. difficile were found in 87% of the pig farms and in 40% of the cattle farms involved in the cross-sectional studies, with a 20% prevalence among suckling piglets and 6.7% prevalence in neonatal calves, with no significant difference between animals with and without diarrheal symptoms. The prevalence of C. difficile in older animal categories was significantly lower. This result suggests that young age is an important risk factor for C. difficile colonization. In cross-sectional studies at slaughterhouses, in both the heavy pigs and dairy cows examined, only 2% of the intestinal content samples were positive for C. difficile and no contamination was found on the surface of the carcasses. Considering passive surveillance, the prevalence rates of positive samples were 29% in piglets and 1.4% in calves. Overall, 267 strains of animal origin and 97 from humans were collected. In total, 39 ribotypes (RTs) were identified, with RT 078 and RT 018 being predominant among animals and humans, respectively. Several RTs overlapped between animals and patients. In particular, RT 569 was identified as an emergent type in our country. Resistance to erythromycin and moxifloxacin was widely diffused among C. difficile strains, regardless of origin. This study supports C. difficile as a pathogen of one-health importance and highlights the need for a collaborative approach between physicians and veterinarians to control and prevent infections that are able to cross species and geographical barriers.
Keywords: CDI; Clostridioides difficile; PCR-ribotyping; animal; antibiotic resistance; food; human; one health.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

