The Volatile Organic Compounds of Streptomyces spp.: An In-Depth Analysis of Their Antifungal Properties
- PMID: 37512992
- PMCID: PMC10384482
- DOI: 10.3390/microorganisms11071820
The Volatile Organic Compounds of Streptomyces spp.: An In-Depth Analysis of Their Antifungal Properties
Abstract
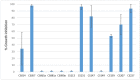
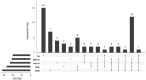
The study of volatile organic compounds (VOCs) has expanded because of the growing need to search for new bioactive compounds that could be used as therapeutic alternatives. These small molecules serve as signals to establish interactions with other nearby organisms in the environment. In this work, we evaluated the antifungal effect of VOCs produced by different Streptomyces spp. This study was performed using VOC chamber devices that allow for the free exchange of VOCs without physical contact between microorganisms or the diffusible compounds they produce. Antifungal activity was tested against Escovopsis weberi, a fungal pathogen that affects ant nest stability, and the results showed that Streptomyces spp. CS014, CS057, CS131, CS147, CS159, CS207, and CS227 inhibit or reduce the fungal growth with their emitted VOCs. A GS-MS analysis of volatiles produced and captured by activated charcoal suggested that these Streptomyces strains synthesize several antifungal VOCs, many of them produced because of the presence of E. weberi, with the accumulation of various VOCs determining the growth inhibition effect.
Keywords: Escovopsis weberi; GS-MS; Streptomyces; antifungal; secondary metabolism; volatile organic compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Hopwood D.A. Streptomyces in Nature and Medicine: The Antibiotic Makers. Oxford University Press; New York, NY, USA: 2007.
-
- Ceniceros A., Cuervo L., Méndez C., Salas J.A., Olano C., Malmierca M.G. A Multidisciplinary Approach to Unraveling the Natural Product Biosynthetic Potential of a Streptomyces Strain Collection Isolated from Leaf-Cutting Ants. Microorganisms. 2021;9:2225. doi: 10.3390/microorganisms9112225. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

