Radiofluorination of an Anionic, Azide-Functionalized Teroligomer by Copper-Catalyzed Azide-Alkyne Cycloaddition
- PMID: 37513105
- PMCID: PMC10385230
- DOI: 10.3390/nano13142095
Radiofluorination of an Anionic, Azide-Functionalized Teroligomer by Copper-Catalyzed Azide-Alkyne Cycloaddition
Abstract
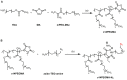
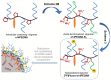
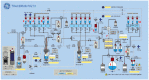
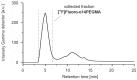
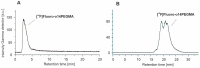
This study describes the synthesis, radiofluorination and purification of an anionic amphiphilic teroligomer developed as a stabilizer for siRNA-loaded calcium phosphate nanoparticles (CaP-NPs). As the stabilizing amphiphile accumulates on nanoparticle surfaces, the fluorine-18-labeled polymer should enable to track the distribution of the CaP-NPs in brain tumors by positron emission tomography after application by convection-enhanced delivery. At first, an unmodified teroligomer was synthesized with a number average molecular weight of 4550 ± 20 Da by free radical polymerization of a defined composition of methoxy-PEG-monomethacrylate, tetradecyl acrylate and maleic anhydride. Subsequent derivatization of anhydrides with azido-TEG-amine provided an azido-functionalized polymer precursor (o14PEGMA-N3) for radiofluorination. The 18F-labeling was accomplished through the copper-catalyzed cycloaddition of o14PEGMA-N3 with diethylene glycol-alkyne-substituted heteroaromatic prosthetic group [18F]2, which was synthesized with a radiochemical yield (RCY) of about 38% within 60 min using a radiosynthesis module. The 18F-labeled polymer [18F]fluoro-o14PEGMA was obtained after a short reaction time of 2-3 min by using CuSO4/sodium ascorbate at 90 °C. Purification was performed by solid-phase extraction on an anion-exchange cartridge followed by size-exclusion chromatography to obtain [18F]fluoro-o14PEGMA with a high radiochemical purity and an RCY of about 15%.
Keywords: 18F-polymer; CuAAC; PEG-[18F]FPyKYNE; click reaction; fluorine-18; teroligomer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Efficient (18)F-labeling of large 37-amino-acid pHLIP peptide analogues and their biological evaluation.Bioconjug Chem. 2012 Aug 15;23(8):1557-66. doi: 10.1021/bc3000222. Epub 2012 Jul 30. Bioconjug Chem. 2012. PMID: 22784215 Free PMC article.
-
Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification.J Vis Exp. 2019 Apr 30;(146). doi: 10.3791/6328. J Vis Exp. 2019. PMID: 31038480
-
Preparation of 18F-labeled peptides using the copper(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition.Nat Protoc. 2011 Oct 13;6(11):1718-25. doi: 10.1038/nprot.2011.390. Nat Protoc. 2011. PMID: 22011654
-
[18F]azadibenzocyclooctyne ([18F]ADIBO): a biocompatible radioactive labeling synthon for peptides using catalyst free [3+2] cycloaddition.Bioorg Med Chem Lett. 2011 Dec 1;21(23):6987-91. doi: 10.1016/j.bmcl.2011.09.126. Epub 2011 Oct 8. Bioorg Med Chem Lett. 2011. PMID: 22024032
-
18F-labeling using click cycloadditions.Biomed Res Int. 2014;2014:361329. doi: 10.1155/2014/361329. Epub 2014 May 27. Biomed Res Int. 2014. PMID: 25003110 Free PMC article. Review.
Cited by
-
Application of Nanomaterials in Biomedical Imaging and Cancer Therapy II.Nanomaterials (Basel). 2024 Oct 11;14(20):1627. doi: 10.3390/nano14201627. Nanomaterials (Basel). 2024. PMID: 39452963 Free PMC article.
-
[18F]-Radiolabelled Nanoplatforms: A Critical Review of Their Intrinsic Characteristics, Radiolabelling Methods, and Purification Techniques.Molecules. 2024 Mar 29;29(7):1537. doi: 10.3390/molecules29071537. Molecules. 2024. PMID: 38611815 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

