Construction of Built-In Electric Field in TiO2@Ti2O3 Core-Shell Heterojunctions toward Optimized Photocatalytic Performance
- PMID: 37513136
- PMCID: PMC10386241
- DOI: 10.3390/nano13142125
Construction of Built-In Electric Field in TiO2@Ti2O3 Core-Shell Heterojunctions toward Optimized Photocatalytic Performance
Abstract
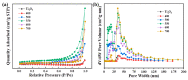
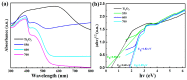
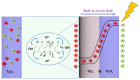
A series of Ti2O3@TiO2 core-shell heterojunction composite photocatalysts with different internal electric fields were synthesized using simple heat treatment methods. The synthesized Ti2O3@TiO2 core-shell heterojunction composites were characterized by means of SEM, XRD, PL, UV-Vis, BET, SPV, TEM and other related analytical techniques. Tetracycline (TC) was used as the degradation target to evaluate the photocatalytic performance of the synthesized Ti2O3@TiO2 core-shell heterojunction composites. The relevant test results show that the photocatalytic performance of the optimized materials has been significantly enhanced compared to Ti2O3, while the photocatalytic degradation rate has increased from 28% to 70.1%. After verification via several different testing and characterization techniques, the excellent catalytic performance is attributed to the efficient separation efficiency of the photogenerated charge carriers derived from the built-in electric field formed between Ti2O3 and TiO2. When the recombination of electrons and holes is occupied, more charges are generated to reach the surface of the photocatalyst, thereby improving the photocatalytic degradation efficiency. Thus, this work provides a universal strategy to enhance the photocatalytic performance of Ti2O3 by coupling it with TiO2 to build an internal electric field.
Keywords: Ti2O3@TiO2 core-shell heterojunction; built-in electric field; photocatalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Wu S., Hu H., Lin Y., Zhang J., Hu Y.H. Visible light photocatalytic degradation of tetracycline over TiO2. Chem. Eng. J. 2020;382:122842. doi: 10.1016/j.cej.2019.122842. - DOI
-
- Xiao Z.-J., Feng X.-C., Shi H.-T., Zhou B.-Q., Wang W.-Q., Ren N.-Q. Why the cooperation of radical and non-radical pathways in PMS system leads to a higher efficiency than a single pathway in tetracycline degradation. J. Hazard. Mater. 2022;424:127247. doi: 10.1016/j.jhazmat.2021.127247. - DOI - PubMed
-
- Zhang Q., Jiang L., Wang J., Zhu Y., Pu Y., Dai W. Photocatalytic degradation of tetracycline antibiotics using three-dimensional network structure perylene diimide supramolecular organic photocatalyst under visible-light irradiation. Appl. Catal. B Environ. 2020;277:119122. doi: 10.1016/j.apcatb.2020.119122. - DOI
-
- Li R., Zhang Y., Deng H., Zhang Z., Wang J.J., Shaheen S.M., Xiao R., Rinklebe J., Xi B., He X., et al. Removing tetracycline and Hg(II) with ball-milled magnetic nanobiochar and its potential on polluted irrigation water reclamation. J. Hazard. Mater. 2020;384:121095. doi: 10.1016/j.jhazmat.2019.121095. - DOI - PubMed
-
- Guo J., Wang L., Wei X., Alothman Z.A., Albaqami M.D., Malgras V., Yamauchi Y., Kang Y., Wang M., Guan W., et al. Direct Z-scheme CuInS2/Bi2MoO6 heterostructure for enhanced photocatalytic degradation of tetracycline under visible light. J. Hazard. Mater. 2021;415:125591. doi: 10.1016/j.jhazmat.2021.125591. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

