Bodipy Dimer for Enhancing Triplet-Triplet Annihilation Upconversion Performance
- PMID: 37513346
- PMCID: PMC10384713
- DOI: 10.3390/molecules28145474
Bodipy Dimer for Enhancing Triplet-Triplet Annihilation Upconversion Performance
Abstract
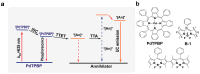
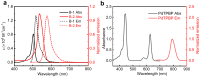
Triplet-triplet annihilation upconversion (TTA-UC) has considerable potential for emerging applications in bioimaging, optogenetics, photoredox catalysis, solar energy harvesting, etc. Fluoroboron dipyrrole (Bodipy) dyes are an essential type of annihilator in TTA-UC. However, conventional Bodipy dyes generally have large molar extinction coefficients and small Stokes shifts (<20 nm), subjecting them to severe internal filtration effects at high concentrations, and resulting in low upconversion quantum efficiency of TTA-UC systems using Bodipy dyes as annihilators. In this study, a Bodipy dimer (B-2) with large Stokes shifts was synthesized using the strategy of dimerization of an already reported Bodipy annihilator (B-1). Photophysical characterization and theoretical chemical analysis showed that both B-1 and B-2 can couple with the red light-activated photosensitizer PdTPBP to fulfill TTA-UC; however, the higher fluorescence quantum yield of B-2 resulted in a higher upconversion efficiency (ηUC) for PdTPBP/B-2 (10.7%) than for PdTPBP/B-1 (4.0%). This study proposes a new strategy to expand Bodipy Stokes shifts and improve TTA-UC performance, which can facilitate the application of TTA-UC in photonics and biophotonics.
Keywords: Stokes shift; boron-dipyrromethene; fluorescence quantum yield; triplet-triplet annihilation upconversion; upconversion quantum efficiency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
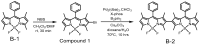


References
-
- Wen S., Zhou J., Schuck P.J., Suh Y.D., Schmidt T.W., Jin D. Future and challenges for hybrid upconversion nanosystems. Nat. Photonics. 2019;13:828–838. doi: 10.1038/s41566-019-0528-x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

