Artemisinin Confers Neuroprotection against 6-OHDA-Induced Neuronal Injury In Vitro and In Vivo through Activation of the ERK1/2 Pathway
- PMID: 37513399
- PMCID: PMC10385954
- DOI: 10.3390/molecules28145527
Artemisinin Confers Neuroprotection against 6-OHDA-Induced Neuronal Injury In Vitro and In Vivo through Activation of the ERK1/2 Pathway
Abstract
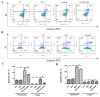
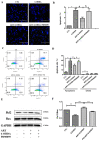
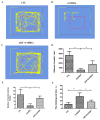
Parkinson's disease (PD) is an age-related, progressive neurodegenerative disease characterized by the gradual and massive loss of dopaminergic neurons in the substantia nigra pars compacta (SNc). We have recently reported that artemisinin, an FDA-approved first-line antimalarial drug, possesses a neuroprotective effect. However, the effects and underlying mechanisms of artemisinin on Parkinson's disease remain to be elucidated. In this study, we investigated the neuroprotective effects of artemisinin on 6-OHDA and MPP+ in neuronal cells and animal models, as well as the underlying mechanisms. Our results showed that artemisinin significantly attenuated the loss of cell viability, LDH release, elevated levels of reactive oxygen species (ROS), the collapse of the mitochondria trans-membrane potential and cell apoptosis in PC12 cells. Western blot results showed that artemisinin stimulated the phosphorylation of ERK1/2, its upstream signaling proteins c-Raf and MEK and its downstream target CREB in PC12 cells in a time- and concentration-dependent manner. In addition, the protective effect of artemisinin was significantly reduced when the ERK pathway was blocked using the ERK pathway inhibitor PD98059 or when the expression of ERK was knocked down using sgRNA. These results indicate the essential role of ERK in the protective effect of artemisinin. Similar results were obtained in SH-SY5Y cells and primary cultured neurons treated with 6-OHDA, as well as in cellular models of MPP+ injury. More interestingly, artemisinin attenuated PD-like behavior deficit in mice injected with 6-OHDA evaluated by behavioral tests including swimming test, pole-test, open field exploration and rotarod tests. Moreover, artemisinin also stimulated the phosphorylation of ERK1/2, inhibited apoptosis, and rescued dopaminergic neurons in SNc of these animals. Application of ERK pathway inhibitor PD98059 blocked the protective effect of artemisinin in mice during testing. Taking these results together, it was indicated that artemisinin preserves neuroprotective effects against 6-OHDA and MPP+ induced injury both in vitro and in vivo by the stimulation of the ERK1/2 signaling pathway. Our findings support the potential therapeutic effect of artemisinin in the prevention and treatment of Parkinson's disease.
Keywords: 6-OHDA; MPTP/MPP+; Parkinson’s disease; apoptosis; artemisinin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





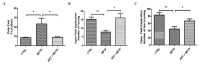



References
MeSH terms
Substances
Grants and funding
- 32070969/National Natural Science Foundation of China
- 0127/2019/A3, 0113/2018/A3 and 0038/2020/AMJ/Science and Technology Development Fund, Macau SAR
- 2022-Natural Science Foundation/Guangdong Provincial Funding Committee for Basic and Applied Fundamental Research
- LY21H280010/Natural Science Foundation of Zhejiang Province
- 2021KY136/Medical and health Science and Technology Project of Zhejiang Province
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

