The Importance of Measuring SARS-CoV-2-Specific T-Cell Responses in an Ongoing Pandemic
- PMID: 37513709
- PMCID: PMC10385870
- DOI: 10.3390/pathogens12070862
The Importance of Measuring SARS-CoV-2-Specific T-Cell Responses in an Ongoing Pandemic
Abstract
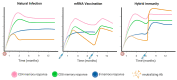
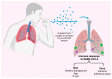
Neutralizing antibodies are considered a correlate of protection against SARS-CoV-2 infection and severe COVID-19, although they are not the only contributing factor to immunity: T-cell responses are considered important in protecting against severe COVID-19 and contributing to the success of vaccination effort. T-cell responses after vaccination largely mirror those of natural infection in magnitude and functional capacity, but not in breadth, as T-cells induced by vaccination exclusively target the surface spike glycoprotein. T-cell responses offer a long-lived line of defense and, unlike humoral responses, largely retain reactivity against the SARS-CoV-2 variants. Given the increasingly recognized role of T-cell responses in protection against severe COVID-19, the circulation of SARS-CoV-2 variants, and the potential implementation of novel vaccines, it becomes imperative to continuously monitor T-cell responses. In addition to "classical" T-cell assays requiring the isolation of peripheral blood mononuclear cells, simple whole-blood-based interferon-γ release assays have a potential role in routine T-cell response monitoring. These assays could be particularly useful for immunocompromised people and other clinically vulnerable populations, where interactions between cellular and humoral immunity are complex. As we continue to live alongside COVID-19, the importance of considering immunity as a whole, incorporating both humoral and cellular responses, is crucial.
Keywords: SARS-CoV-2; T-cell response; cellular immunity; humoral immunity; immunocompromised; interferon-γ release assay; vaccination.
Conflict of interest statement
DG has received consulting fees from Eli Lilly, PDB Biotech, and Quidel; she has received payment or honoraria from Amgen, Almirall, Biogen, bioMérieux, Celgene, DiaSorin, Janssen Biotech, and QIAGEN; and she has participated in data safety monitoring boards or advisory boards for Celgene and Eli Lilly. LP has no conflicts of interest to report. AS has received grants from the Bill & Melinda Gates Foundation, the National Institute of Allergy and Infectious Diseases, and the National Institutes of Health; he has received consulting fees from AstraZeneca, Avalia Immunotherapies, Flow Pharma, Fortress, Gerson Lehrman Group, Gilead, Gritstone bio, Guggenheim, MEDACorp, Merck, Moderna, NA Vaccine Institute, QIAGEN, Rivervest, Sanofi, and Turnstone; he has received reimbursement for travel expenses from AstraZeneca, Aviara, Harvard, HLA, Keystone Symposia, Massachusetts General Hospital, Moderna, Ohio State University, Oxford University, Periscope, University of Oporto, and WVC; and he has filed for patent protection for various aspects of T-cell epitope and vaccine design work. RdV has no conflicts of interest to report.
Figures

References
-
- Geers D., Shamier M.C., Bogers S., den Hartog G., Gommers L., Nieuwkoop N.N., Schmitz K.S., Rijsbergen L.C., van Osch J.A.T., Dijkhuizen E., et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci. Immunol. 2021;6:eabj1750. doi: 10.1126/sciimmunol.abj1750. - DOI - PMC - PubMed
-
- Fiolet T., Kherabi Y., MacDonald C., Ghosn J., Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022;28:202–221. doi: 10.1016/j.cmi.2021.10.005. - DOI - PMC - PubMed
-
- World Health Organization Coronfavirus Disease (COVID-19): Vaccines. [(accessed on 22 June 2022)]. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-dis....
-
- Garcia-Beltran W.F., St Denis K.J., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., Berrios C., Ofoman O., Chang C.C., Hauser B.M., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457–466.e4. doi: 10.1016/j.cell.2021.12.033. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

