The Potential of Probiotics as Ingestible Adjuvants and Immune Modulators for Antiviral Immunity and Management of SARS-CoV-2 Infection and COVID-19
- PMID: 37513775
- PMCID: PMC10384479
- DOI: 10.3390/pathogens12070928
The Potential of Probiotics as Ingestible Adjuvants and Immune Modulators for Antiviral Immunity and Management of SARS-CoV-2 Infection and COVID-19
Abstract
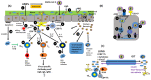
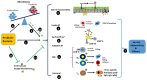
Probiotic bacteria are able to modulate general antiviral responsiveness, including barrier functionality and innate and adaptive immune responses. The COVID-19 pandemic, resulting from SARS-CoV-2 infection, has created a need to control and treat this viral infection and its ensuing immunopathology with a variety of approaches; one such approach may involve the administration of probiotic bacteria. As with most viral infections, its pathological responses are not fully driven by the virus, but are significantly contributed to by the host's immune response to viral infection. The potential adoption of probiotics in the treatment of COVID-19 will have to appreciate the fine line between inducing antiviral immunity without over-provoking immune inflammatory responses resulting in host-derived immunopathological tissue damage. Additionally, the effect exerted on the immune system by SARS-CoV-2 evasion strategies will also have to be considered when developing a robust response to this virus. This review will introduce the immunopathology of COVID-19 and the immunomodulatory effects of probiotic strains, and through their effects on a range of respiratory pathogens (IAV, SARS-CoV, RSV), as well as SARS-CoV-2, will culminate in a focus on how these bacteria can potentially manipulate both infectivity and immune responsiveness via barrier functionality and both innate and adaptive immunity. In conclusion, the harnessing of induction and augmentation of antiviral immunity via probiotics may not only act as an ingestible adjuvant, boosting immune responsiveness to SARS-CoV-2 infection at the level of barrier integrity and innate and adaptive immunity, but also act prophylactically to prevent infection and enhance protection afforded by current vaccine regimens.
Keywords: COVID-19; SARS-CoV-2; antiviral immunity; immune evasion; probiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Richman D.D., Whitley R.J., Hayden F.G. Clinical Virology. 4th ed. ASM Press; Washington, DC, USA: 2016.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

