Indole-Acrylonitrile Derivatives as Potential Antitumor and Antimicrobial Agents-Synthesis, In Vitro and In Silico Studies
- PMID: 37513830
- PMCID: PMC10386429
- DOI: 10.3390/ph16070918
Indole-Acrylonitrile Derivatives as Potential Antitumor and Antimicrobial Agents-Synthesis, In Vitro and In Silico Studies
Abstract
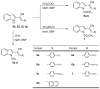
A series of 2-(1H-indol-2-yl)-3-acrylonitrile derivatives, 2a-x, 3, 4a-b, 5a-d, 6a-b, and 7, were synthesized as potential antitumor and antimicrobial agents. The structures of the prepared compounds were evaluated based on elemental analysis, IR, 1H- and 13NMR, as well as MS spectra. X-ray crystal analysis of the representative 2-(1H-indol-2-yl)-3-acrylonitrile 2l showed that the acrylonitrile double bond was Z-configured. All compounds were screened at the National Cancer Institute (USA) for their activities against a panel of approximately 60 human tumor cell lines and the relationship between structure and in vitro antitumor activity is discussed. Compounds of interest 2l and 5a-d showed significant growth inhibition potency against various tumor cell lines with the mean midpoint GI50 values of all tests in the range of 0.38-7.91 μM. The prominent compound with remarkable activity (GI50 = 0.0244-5.06 μM) and high potency (TGI = 0.0866-0.938 μM) against some cell lines of leukemia (HL-60(TB)), non-small cell lung cancer (NCI-H522), colon cancer (COLO 205), CNS cancer (SF-539, SNB-75), ovarian cancer ((OVCAR-3), renal cancer (A498, RXF 393), and breast cancer (MDA-MB-468) was 3-[4-(dimethylamino)phenyl]-2-(1-methyl-1H-indol-2-yl)acrylonitrile (5c). Moreover, the selected 2-(1H-indol-2-yl)-3-acrylonitriles 2a-c and 2e-x were evaluated for their antibacterial and antifungal activities against Gram-positive and Gram-negative pathogens as well as Candida albicans. Among them, 2-(1H-indol-2-yl)-3-(1H-pyrrol-2-yl)acrylonitrile (2x) showed the most potent antimicrobial activity and therefore it can be considered as a lead structure for further development of antimicrobial agents. Finally, molecular docking studies as well as drug-likeness and ADME profile prediction were carried out.
Keywords: ADME; antimicrobial activity; cell growth inhibition; indole-acrylonitrile derivatives; molecular docking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





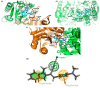



References
-
- Kumar S., Ritika A brief review of the biological potential of indole derivatives. Future J. Pharm. Sci. 2020;6:121. doi: 10.1186/s43094-020-00141-y. - DOI
-
- Kumar D., Sharma S., Kalra S., Singh G., Monga V., Kumar B. Medicinal perspective of indole derivatives: Recent developments and structure-activity relationship studies. Curr. Drug Targets. 2020;21:864–891. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

