Combining Molecular Dynamics Simulations and Biophysical Characterization to Investigate Protein-Specific Excipient Effects on Reteplase during Freeze Drying
- PMID: 37514040
- PMCID: PMC10384596
- DOI: 10.3390/pharmaceutics15071854
Combining Molecular Dynamics Simulations and Biophysical Characterization to Investigate Protein-Specific Excipient Effects on Reteplase during Freeze Drying
Abstract
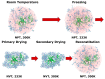
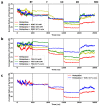
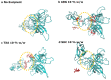
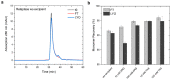
We performed molecular dynamics simulations of Reteplase in the presence of different excipients to study the stabilizing mechanisms and to identify the role of excipients during freeze drying. To simulate the freeze-drying process, we divided the process into five distinct steps: (i) protein-excipient formulations at room temperature, (ii) the ice-growth process, (iii)-(iv) the partially solvated and fully dried formulations, and (v) the reconstitution. Furthermore, coarse-grained (CG) simulations were employed to explore the protein-aggregation process in the presence of arginine. By using a coarse-grained representation, we could observe the collective behavior and interactions between protein molecules during the aggregation process. The CG simulations revealed that the presence of arginine prevented intermolecular interactions of the catalytic domain of Reteplase, thus reducing the aggregation propensity. This suggests that arginine played a stabilizing role by interacting with protein-specific regions. From the freeze-drying simulations, we could identify several protein-specific events: (i) collapse of the domain structure, (ii) recovery of the drying-induced damages during reconstitution, and (iii) stabilization of the local aggregation-prone region via direct interactions with excipients. Complementary to the simulations, we employed nanoDSF, size-exclusion chromatography, and CD spectroscopy to investigate the effect of the freeze-drying process on the protein structure and stability.
Keywords: Reteplase; arginine; coarse-grained simulations; formulation development; freeze drying; molecular dynamics; protein stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Seifert I., Friess W. Freeze Drying of Pharmaceutical Products. CRC Press; Boca Raton, FL, USA: 2019. 3 Established and Novel Excipients for Freeze-Drying of Proteins; p. 33.
LinkOut - more resources
Full Text Sources

