Synthetic Pathways to Non-Psychotropic Phytocannabinoids as Promising Molecules to Develop Novel Antibiotics: A Review
- PMID: 37514074
- PMCID: PMC10384972
- DOI: 10.3390/pharmaceutics15071889
Synthetic Pathways to Non-Psychotropic Phytocannabinoids as Promising Molecules to Develop Novel Antibiotics: A Review
Abstract
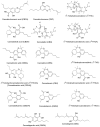
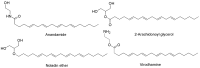
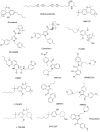
Due to the rapid emergence of multi drug resistant (MDR) pathogens against which current antibiotics are no longer functioning, severe infections are becoming practically untreatable. Consequently, the discovery of new classes of effective antimicrobial agents with novel mechanism of action is becoming increasingly urgent. The bioactivity of Cannabis sativa, an herbaceous plant used for millennia for medicinal and recreational purposes, is mainly due to its content in phytocannabinoids (PCs). Among the 180 PCs detected, cannabidiol (CBD), Δ8 and Δ9-tetrahydrocannabinols (Δ8-THC and Δ9-THC), cannabichromene (CBC), cannabigerol (CBG), cannabinol (CBN) and some of their acidic precursors have demonstrated from moderate to potent antibacterial effects against Gram-positive bacteria (MICs 0.5-8 µg/mL), including methicillin-resistant Staphylococcus aureus (MRSA), epidemic MRSA (EMRSA), as well as fluoroquinolone and tetracycline-resistant strains. Particularly, the non-psychotropic CBG was also capable to inhibit MRSA biofilm formation, to eradicate even mature biofilms, and to rapidly eliminate MRSA persiter cells. In this scenario, CBG, as well as other minor non-psychotropic PCs, such as CBD, and CBC could represent promising compounds for developing novel antibiotics with high therapeutic potential. Anyway, further studies are necessary, needing abundant quantities of such PCs, scarcely provided naturally by Cannabis plants. Here, after an extensive overture on cannabinoids including their reported antimicrobial effects, aiming at easing the synthetic production of the necessary amounts of CBG, CBC and CBD for further studies, we have, for the first time, systematically reviewed the synthetic pathways utilized for their synthesis, reporting both reaction schemes and experimental details.
Keywords: Cannabis sativa; bacterial resistance; cannabichromene (CBC); cannabidiol (CBC); cannabigerol (CBG); endocannabinois (ECs); methicillin-resistant S. aureus (MRSA); multi drug resistant (MDR) bacteria; phytocannabinoids (PCs); synthetic cannabinoids (SCs); synthetic procedures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







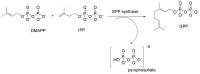
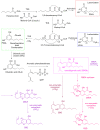











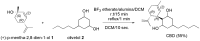




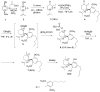






References
-
- WHO Antimicrobial Resistance. [(accessed on 3 May 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

