Co-Encapsulation of Curcumin and α-Tocopherol in Bicosome Systems: Physicochemical Properties and Biological Activity
- PMID: 37514098
- PMCID: PMC10383532
- DOI: 10.3390/pharmaceutics15071912
Co-Encapsulation of Curcumin and α-Tocopherol in Bicosome Systems: Physicochemical Properties and Biological Activity
Abstract
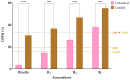
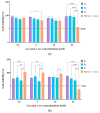
A novel co-encapsulation system called bicosomes (bicelles within liposomes) has been developed to overcome the limitations associated with the topical application of curcumin (cur) and α-tocopherol (α-toc). The physicochemical properties and biological activity in vitro of bicosome systems were evaluated. Bicelles were prepared with DPPC, DHPC, cur, and α-toc (cur/α-toc-bicelles). Liposomal vesicles loading cur/α-toc-bicelles were prepared with Lipoid P-100 and cholesterol-forming cur/α-toc-bicosomes. Three cur/α-toc-bicosomes were evaluated using different total lipid percentages (12, 16, and 20% w/v). The results indicated that formulations manage to solubilize cur and α-toc in homogeneous bicelles < 20 nm, while the bicosomes reaches 303-420 nm depending on the total lipid percentage in the systems. Bicosomes demonstrated high-encapsulation efficiency (EE) for cur (56-77%) and α-toc (51-65%). The loading capacity (LC) for both antioxidant compounds was 52-67%. In addition, cur/α-toc-bicosomes decreased the lipid oxidation by 52% and increased the antioxidant activity by 60% compared to unloaded bicosomes. The cell viability of these cur/α-toc-bicosomes was >85% in fibroblasts (3T3L1/CL-173™) and ≥65% in keratinocytes (Ha-CaT) and proved to be hematologically compatible. The cur/α-toc-bicelles and cur/α-toc-bicosomes inhibited the growth of C. albicans in a range between 33 and 76%. Our results propose bicosome systems as a novel carrier able to co-encapsulate, solubilize, protect, and improve the delivery performance of antioxidant molecules. The relevance of these findings is based on the synergistic antioxidant effect of its components, its biocompatibility, and its efficacy for dermal tissue treatment damaged by oxidative stress or by the presence of C. albicans. However, further studies are needed to assess the efficacy and safety of cur/α-toc bicosomes in vitro and in vivo.
Keywords: bicosomes; candidiasis; curcumin; delivery system; oxidative stress; skin; α-tocopherol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Jing Y., Ruan L., Jiang G., Nie L., Shavandi A., Sun Y., Xu J., Shao X., Zhu J. Regenerated silk fibroin and alginate composite hydrogel dressings loaded with curcumin nanoparticles for bacterial-infected wound closure. Biomater. Res. 2023;149:213405. doi: 10.1016/j.bioadv.2023.213405. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

