Delicate Hybrid Laponite-Cyclic Poly(ethylene glycol) Nanoparticles as a Potential Drug Delivery System
- PMID: 37514184
- PMCID: PMC10384068
- DOI: 10.3390/pharmaceutics15071998
Delicate Hybrid Laponite-Cyclic Poly(ethylene glycol) Nanoparticles as a Potential Drug Delivery System
Abstract
The objective of the study was to explore the feasibility of a new drug delivery system using laponite (LAP) and cyclic poly(ethylene glycol) (cPEG). Variously shaped and flexible hybrid nanocrystals were made by both the covalent and physical attachment of chemically homogeneous cyclized PEG to laponite nanodisc plates. The size of the resulting, nearly spherical particles ranged from 1 to 1.5 µm, while PEGylation with linear methoxy poly (ethylene glycol) (mPEG) resulted in fragile sheets of different shapes and sizes. When infused with 10% doxorubicin (DOX), a drug commonly used in the treatment of various cancers, the LAP-cPEG/DOX formulation was transparent and maintained liquid-like homogeneity without delamination, and the drug loading efficiency of the LAP-cPEG nano system was found to be higher than that of the laponite-poly(ethylene glycol) LAP-mPEG system. Furthermore, the LAP-cPEG/DOX formulation showed relative stability in phosphate-buffered saline (PBS) with only 15% of the drug released. However, in the presence of human plasma, about 90% of the drug was released continuously over a period of 24 h for the LAP-cPEG/DOX, while the LAP-mPEG/DOX formulation released 90% of DOX in a 6 h burst. The results of the cell viability assay indicated that the LAP-cPEG/DOX formulation could effectively inhibit the proliferation of A549 lung carcinoma epithelial cells. With the DOX concentration in the range of 1-2 µM in the LAP-cPEG/DOX formulation, enhanced drug effects in both A549 lung carcinoma epithelial cells and primary lung epithelial cells were observed compared to LAP-mPEG/DOX. The unique properties and effects of cPEG nanoparticles provide a potentially better drug delivery system and generate interest for further targeting studies and applications.
Keywords: PEGylation of laponite; drug delivery; functional cyclized polyethylene glycol; hybrid nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

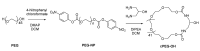
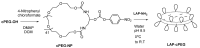











References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

