Solarplast®-An Enzymatically Treated Spinach Extract
- PMID: 37514292
- PMCID: PMC10384499
- DOI: 10.3390/plants12142678
Solarplast®-An Enzymatically Treated Spinach Extract
Abstract
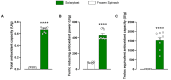
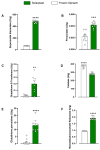
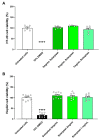
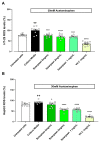
In the modern world we are constantly bombarded by environmental and natural stimuli that can result in oxidative stress. Antioxidant molecules and enzymes help the human body scavenge reactive oxygen species and prevent oxidative damage. Most organisms possess intrinsic antioxidant activity, but also benefit from the consumption of antioxidants from their diet. Leafy green vegetables such as spinach are a well-researched rich source of dietary antioxidant molecules. However, plant cell walls are difficult to digest for many individuals and the bio-accessibility of nutrients and antioxidants from these sources can be limited by the degree of digestion and assimilation. Through a specific enzymatic process, Solarplast® contains organic spinach protoplasts without the cell wall, which may facilitate higher yield and efficacy of beneficial antioxidant molecules. In this study, analytical techniques coupled to in vitro bioassays were used to determine the potential antioxidant activity of Solarplast® and determine its antioxidant enzymatic capabilities. Solarplast® demonstrated superior antioxidant activity when compared to frozen spinach leaves in TOC, FRAP and TEAC antioxidant assays. Several antioxidant enzymes were also increased in Solarplast®, when compared to frozen spinach. As a functional readout, Solarplast® attenuated hydrogen peroxide-, ethanol- and acetaminophen-induced increases in oxidative stress and cytotoxicity in both intestinal (HT-29) and liver (HepG2) cell lines. These findings suggest that Solarplast® may represent a non-GMO, plant-based food supplement to help reduce oxidative stress in the human body.
Keywords: HT-29; HepG2; Solarplast®; antioxidant; oxidative stress; spinach.
Conflict of interest statement
Annie Simon, Shahneela Mazhar, Ekaterina Khokhlova, Natasha Leeuwendaal, Christopher Phipps, John Deaton, Kieran Rea and Joan Colom are employed by ADM Deerland Probiotics & Enzymes and ADM Cork H&W Limited. All authors declare no other competing interests. The authors are all ADM employees and contributed to designing the study; performing the analyses; interpreting the data; in the writing of the manuscript; and in the decision to publish the results.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

