Sol-Gel Approach for Fabricating Silica/Epoxy Nanocomposites
- PMID: 37514377
- PMCID: PMC10383508
- DOI: 10.3390/polym15142987
Sol-Gel Approach for Fabricating Silica/Epoxy Nanocomposites
Abstract
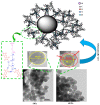
This review focuses on the opportunities provided by sol-gel chemistry for the production of silica/epoxy nanocomposites, with significant representative examples of the "extra situ" approach and an updated description of the "in situ" strategy. The "extra situ" strategy enables the creation of nanocomposites containing highly engineered nanoparticles. The "in situ" approach is a very promising synthesis route that allows us to produce, in a much easier and eco-friendly manner, properly flame-retarded silica/epoxy nanocomposites endowed with very interesting properties. The review highlights the recently proposed mechanism of nanoparticles formation, which is expected to help to design the synthesis strategies of nanocomposites, changing their composition (both for the nanoparticle and matrix nature) and with in situ-generated nanoparticles possibly more complex than the ones obtained, until today, through this route.
Keywords: epoxy; flame retardancy; highly engineered nanoparticles; nanocomposites; silica in situ formation mechanism; sol–gel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

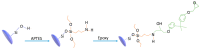











References
-
- Gu H., Ma C., Gu J., Guo J., Yan X., Huang J., Zhang Q., Guo Z. An overview of multifunctional epoxy nanocomposites. J. Mater. Chem. C. 2016;4:5890–5906. doi: 10.1039/C6TC01210H. - DOI
-
- Serra A., Ramis X., Fernández-Francos X. Epoxy Sol–Gel Hybrid Thermosets. Coatings. 2016;6:8. doi: 10.3390/coatings6010008. - DOI
-
- Chu P., Zhang H., Zhao J., Gao F., Guo Y., Dang B., Zhang Z. On the volume resistivity of silica nanoparticle filled epoxy with different surface modifications. Compos. Part A Appl. Sci. Manuf. 2017;99:139–148. doi: 10.1016/j.compositesa.2017.03.036. - DOI
-
- Li H., Wang C., Guo Z., Wang H., Zhang Y., Hong R., Peng Z. Effects of silane coupling agents on the electrical properties of silica/epoxy nanocomposites; Proceedings of the IEEE International Conference on Dielectrics; Montpellier, France. 3–7 July 2016; - DOI
Publication types
LinkOut - more resources
Full Text Sources

