Effect of Drug-Polymer Interaction in Amorphous Solid Dispersion on the Physical Stability and Dissolution of Drugs: The Case of Alpha-Mangostin
- PMID: 37514423
- PMCID: PMC10384849
- DOI: 10.3390/polym15143034
Effect of Drug-Polymer Interaction in Amorphous Solid Dispersion on the Physical Stability and Dissolution of Drugs: The Case of Alpha-Mangostin
Abstract
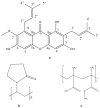
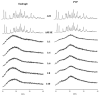
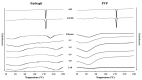
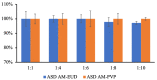
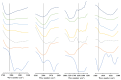
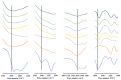
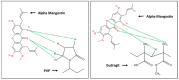
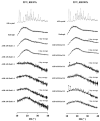
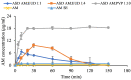
Improving drug solubility is necessary for formulations of poorly water-soluble drugs, especially for oral administration. Amorphous solid dispersions (ASDs) are widely used in the pharmaceutical industry to improve the physical stability and solubility of drugs. Therefore, this study aims to characterize interaction between a drug and polymer in ASD, as well as evaluate the impact on the physical stability and dissolution of alpha-mangostin (AM). AM was used as a model of a poorly water-soluble drug, while polyvinylpyrrolidone (PVP) and eudragit were used as polymers. The amorphization of AM-eudragit and AM-PVP was confirmed as having a halo pattern with powder X-ray diffraction measurements and the absence of an AM melting peak in the differential scanning calorimetry (DSC) curve. The solubility of amorphous AM increased in the presence of either eudragit or PVP due to amorphization and interactions of AM-polymer. Furthermore, FT-IR spectroscopy and in silico studies revealed hydrogen bond interactions between the carbonyl group of AM and the proton of eudragit as well as PVP. AM-eudragit with a ratio of 1:1 recrystallized after 7 days of storage at 25 °C and 90% RH, while the AM-PVP 1:4 and 1:10 samples retained the X-ray halo patterns, even under humid conditions. In a dissolution test, the presence of polymer in ASD significantly improved the dissolution profile due to the intermolecular interaction of AM-polymer. AM-eudragit 1:4 maintained AM supersaturation for a longer time compared to the 1:1 sample. However, a high supersaturation was not achieved in AM-PVP 1:10 due to the formation of large agglomerations, leading to a slow dissolution rate. Based on the results, interaction of AM-polymer in ASD can significantly improve the pharmaceutical properties of AM including the physical stability and dissolution.
Keywords: alpha-mangostin; amorphous solid dispersion; dissolution; hydrogen bond; physical stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Characterization of Alpha Mangostin Loaded-Mesoporous Silica Nanoparticle and the Impact on Dissolution and Physical Stability.Nanotechnol Sci Appl. 2025 Jan 9;18:1-13. doi: 10.2147/NSA.S499007. eCollection 2025. Nanotechnol Sci Appl. 2025. PMID: 39811759 Free PMC article.
-
Inhibition of Crystal Nucleation and Growth in Aqueous Drug Solutions: Impact of Different Polymers on the Supersaturation Profiles of Amorphous Drugs-The Case of Alpha-Mangostin.Pharmaceutics. 2022 Nov 5;14(11):2386. doi: 10.3390/pharmaceutics14112386. Pharmaceutics. 2022. PMID: 36365204 Free PMC article.
-
Characterization and stability of solid dispersions based on PEG/polymer blends.Int J Pharm. 2010 May 10;390(2):165-73. doi: 10.1016/j.ijpharm.2010.01.039. Epub 2010 Feb 10. Int J Pharm. 2010. PMID: 20132875
-
Effects of Additives on the Physical Stability and Dissolution of Polymeric Amorphous Solid Dispersions: a Review.AAPS PharmSciTech. 2023 Aug 21;24(7):175. doi: 10.1208/s12249-023-02622-8. AAPS PharmSciTech. 2023. PMID: 37603110 Review.
-
Advancing Drug Delivery Paradigms: Polyvinyl Pyrolidone (PVP)-Based Amorphous Solid Dispersion for Enhanced Physicochemical Properties and Therapeutic Efficacy.Polymers (Basel). 2024 Jan 20;16(2):286. doi: 10.3390/polym16020286. Polymers (Basel). 2024. PMID: 38276694 Free PMC article. Review.
Cited by
-
The Excellent Chemical Interaction Properties of Poloxamer and Pullulan with Alpha Mangostin on Amorphous Solid Dispersion System: Molecular Dynamics Simulation.Polymers (Basel). 2024 Oct 31;16(21):3065. doi: 10.3390/polym16213065. Polymers (Basel). 2024. PMID: 39518274 Free PMC article.
-
Curcumin Solubility and Bioactivity Enhancement Through Amorphization with Tryptophan via Supercritical Fluid Technology.Int J Mol Sci. 2025 Jan 20;26(2):855. doi: 10.3390/ijms26020855. Int J Mol Sci. 2025. PMID: 39859569 Free PMC article.
-
Amorphous Solid Dispersions of Glycyrrhetinic Acid: Using Soluplus, PVP, and PVPVA as the Polymer Matrix to Enhance Solubility, Bioavailability, and Stability.AAPS PharmSciTech. 2024 Dec 21;26(1):18. doi: 10.1208/s12249-024-03007-1. AAPS PharmSciTech. 2024. PMID: 39707118
-
Enhanced Antioxidant and Neuroprotective Properties of Pterostilbene (Resveratrol Derivative) in Amorphous Solid Dispersions.Int J Mol Sci. 2024 Feb 28;25(5):2774. doi: 10.3390/ijms25052774. Int J Mol Sci. 2024. PMID: 38474022 Free PMC article.
-
Biobran-loaded core/shell nanofibrous scaffold: a promising wound dressing candidate.RSC Adv. 2024 Feb 7;14(7):4930-4945. doi: 10.1039/d3ra08609g. eCollection 2024 Jan 31. RSC Adv. 2024. PMID: 38327812 Free PMC article.
References
-
- Ku M.S., Dulin W. A biopharmaceutical classification-based Right-First-Time formulation approach to reduce human pharmacokinetic variability and project cycle time from First-In-Human to clinical Proof-Of-Concept. Pharm. Dev. Technol. 2012;17:285–302. doi: 10.3109/10837450.2010.535826. - DOI - PubMed
-
- Okada H., Ueda K., Yasuda Y., Higashi K., Inoue M., Ito M., Noguchi S., Kawakami K., Moribe K. Correlation between drug dissolution and resistance to water-induced phase separation in solid dispersion formulations revealed by solid-state NMR spectroscopy. Int. J. Pharm. 2020;577:119086. doi: 10.1016/j.ijpharm.2020.119086. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

